The maturase HydF enables [FeFe] hydrogenase assembly via transient, cofactor-dependent interactions
- PMID: 32620553
- PMCID: PMC7450098
- DOI: 10.1074/jbc.RA119.011419
The maturase HydF enables [FeFe] hydrogenase assembly via transient, cofactor-dependent interactions
Abstract
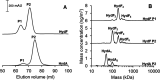
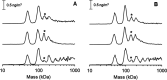
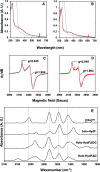
[FeFe] hydrogenases have attracted extensive attention in the field of renewable energy research because of their remarkable efficiency for H2 gas production. H2 formation is catalyzed by a biologically unique hexanuclear iron cofactor denoted the H-cluster. The assembly of this cofactor requires a dedicated maturation machinery including HydF, a multidomain [4Fe4S] cluster protein with GTPase activity. HydF is responsible for harboring and delivering a precatalyst to the apo-hydrogenase, but the details of this process are not well understood. Here, we utilize gas-phase electrophoretic macromolecule analysis to show that a HydF dimer forms a transient interaction complex with the hydrogenase and that the formation of this complex depends on the cofactor content on HydF. Moreover, Fourier transform infrared, electron paramagnetic resonance, and UV-visible spectroscopy studies of mutants of HydF show that the isolated iron-sulfur cluster domain retains the capacity for binding the precatalyst in a reversible fashion and is capable of activating apo-hydrogenase in in vitro assays. These results demonstrate the central role of the iron-sulfur cluster domain of HydF in the final stages of H-cluster assembly, i.e. in binding and delivering the precatalyst.
Keywords: Fourier transform IR (FTIR); chaperone; cofactor; hydrogenase; iron-sulfur protein; mass spectrometry (MS); metal ion–protein interaction; metallo-cofactor assembly; metalloenzyme; protein–protein interaction; scaffold.
© 2020 Németh et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


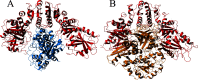

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

