Mechanisms of heart valve development and disease
- PMID: 32620577
- PMCID: PMC7338271
- DOI: 10.1242/dev.183020
Mechanisms of heart valve development and disease
Abstract
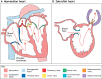
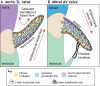
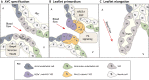
The valves of the heart are crucial for ensuring that blood flows in one direction from the heart, through the lungs and back to the rest of the body. Heart valve development is regulated by complex interactions between different cardiac cell types and is subject to blood flow-driven forces. Recent work has begun to elucidate the important roles of developmental pathways, valve cell heterogeneity and hemodynamics in determining the structure and function of developing valves. Furthermore, this work has revealed that many key genetic pathways involved in cardiac valve development are also implicated in diseased valves. Here, we review recent discoveries that have furthered our understanding of the molecular, cellular and mechanosensitive mechanisms of valve development, and highlight new insights into congenital and acquired valve disease.
Keywords: Congenital valve disease; Heart valve development; Hemodynamics.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Aikawa E., Whittaker P., Farber M., Mendelson K., Padera R. F., Aikawa M. and Schoen F. J. (2006). Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113, 1344-1352. 10.1161/CIRCULATIONAHA.105.591768 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

