Reduced accuracy of MRI deep grey matter segmentation in multiple sclerosis: an evaluation of four automated methods against manual reference segmentations in a multi-center cohort
- PMID: 32621103
- PMCID: PMC7674567
- DOI: 10.1007/s00415-020-10023-1
Reduced accuracy of MRI deep grey matter segmentation in multiple sclerosis: an evaluation of four automated methods against manual reference segmentations in a multi-center cohort
Abstract
Background: Deep grey matter (DGM) atrophy in multiple sclerosis (MS) and its relation to cognitive and clinical decline requires accurate measurements. MS pathology may deteriorate the performance of automated segmentation methods. Accuracy of DGM segmentation methods is compared between MS and controls, and the relation of performance with lesions and atrophy is studied.
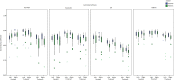
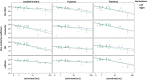
Methods: On images of 21 MS subjects and 11 controls, three raters manually outlined caudate nucleus, putamen and thalamus; outlines were combined by majority voting. FSL-FIRST, FreeSurfer, Geodesic Information Flow and volBrain were evaluated. Performance was evaluated volumetrically (intra-class correlation coefficient (ICC)) and spatially (Dice similarity coefficient (DSC)). Spearman's correlations of DSC with global and local lesion volume, structure of interest volume (ROIV), and normalized brain volume (NBV) were assessed.
Results: ICC with manual volumes was mostly good and spatial agreement was high. MS exhibited significantly lower DSC than controls for thalamus and putamen. For some combinations of structure and method, DSC correlated negatively with lesion volume or positively with NBV or ROIV. Lesion-filling did not substantially change segmentations.
Conclusions: Automated methods have impaired performance in patients. Performance generally deteriorated with higher lesion volume and lower NBV and ROIV, suggesting that these may contribute to the impaired performance.
Keywords: Atrophy; Automated segmentation methods; Deep grey matter; Multiple sclerosis.
Conflict of interest statement
AdS is employed on a project sponsored by a research grant from Teva Pharmaceuticals (grant to H. Vrenken and F. Barkhof). TV has no disclosures. YL has no disclosures related to this paper. JB has no disclosures. JS has no disclosures. SR received fees as invited speaker or travel expenses for attending meeting from Biogen, Merck-Serono, Teva, Sanofi, Novartis. MP has no disclosures. IB is partly employed on projects sponsored by research grants from Teva Pharmaceuticals and Novartis Pharma (grants to HV and FB). AV has no disclosures. VW has no disclosures related to this paper. SR has no disclosures related to this paper. MAR received speakers’ honoraria from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva, Merck Serono, Roche and Celgene and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. CG received fees as speaker for Bayer-Schering Pharma, Sanofi-Aventis, Genzyme, Biogen, Teva, Novartis, and Merck-Serono, and received a grant for research by Teva. AG has no disclosures related to this paper. MCY has no disclosures. AR has no disclosures related to this paper. CE has no disclosures related to this paper. MF is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). NDS has no disclosures related to this paper. LK has no disclosures related to this paper. JLF declares personal fees from Biogen Idec, Merck Serono and Sanofi-Aventis, participation in scientific advisory boards for Almiral, Genzyme and Novartis, personal fees (speaker honoraria) from Biogen, Merck, Santhera and Teva, all unrelated to this paper. FB has received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Teva, Bracco and IXICO. CRGG has received support from the National Multiple Sclerosis Society, the International Progressive Multiple Sclerosis Alliance, the U.S. Office for Naval Research, Mobilengine (free use of platform and programming by Mobilengine Engineers), NIH, as well as travel support from Roche Pharmaceuticals; C.R.G owns stock in Roche, Novartis, GSK, Alnylam, Protalix Biotherapeutics, Arrowhead Pharmaceuticals, Cocrystal Pharma, Sangamo Therapeutics. HV has received research grants from Pfizer, MerckSerono, Novartis and Teva, speaker honoraria from Novartis, and consulting fees from MerckSerono; all funds were paid directly to his Institution.
Figures

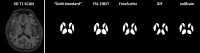

References
-
- Amiri H, de Sitter A, Bendfeldt K, Battaglini M, Gandini Wheeler-Kingshott CAM, Calabrese M, et al. Urgent challenges in quantification and interpretation of brain grey matter atrophy in individual MS patients using MRI. Neuroimage Clin. 2018;19:466–475. doi: 10.1016/j.nicl.2018.04.023. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

