Homologation of the Fischer Indolization: A Quinoline Synthesis via Homo-Diaza-Cope Rearrangement
- PMID: 32621795
- PMCID: PMC7693176
- DOI: 10.1002/anie.202005798
Homologation of the Fischer Indolization: A Quinoline Synthesis via Homo-Diaza-Cope Rearrangement
Abstract
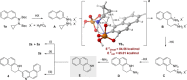
We disclose a new Brønsted acid promoted quinoline synthesis, proceeding via homo-diaza-Cope rearrangement of N-aryl-N'-cyclopropyl hydrazines. Our strategy can be considered a homologation of Fischer's classical indole synthesis and delivers 6-membered N-heterocycles, including previously inaccessible pyridine derivatives. This approach can also be used as a pyridannulation methodology toward constructing polycyclic polyheteroaromatics. A computational analysis has been employed to probe plausible activation modes and to interrogate the role of the catalyst.
Keywords: DFT calculations; Fischer indolization; annulation; cyclopropane; quinolines; rearrangements.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Hou C., Chen P., Liu G., Angew. Chem. Int. Ed. 2020, 59, 2735–2739; - PubMed
- Angew. Chem. 2020, 132, 2757–2761;
-
- Jin Z., Wang X., Huang H., Liang X., Ye J., Org. Lett. 2011, 13, 564–567; - PubMed
-
- Shanahan R. M., Hickey A., Bateman L. M., Light M. E., McGlacken G. P., J. Org. Chem. 2020, 85, 2585–2596; - PubMed
Publication types
LinkOut - more resources
Full Text Sources

