METTL3 Promotes Tumorigenesis and Metastasis through BMI1 m6A Methylation in Oral Squamous Cell Carcinoma
- PMID: 32621798
- PMCID: PMC7544972
- DOI: 10.1016/j.ymthe.2020.06.024
METTL3 Promotes Tumorigenesis and Metastasis through BMI1 m6A Methylation in Oral Squamous Cell Carcinoma
Abstract
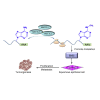
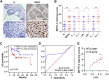
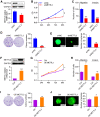
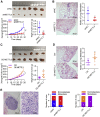
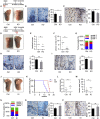
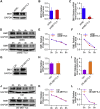
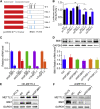
RNA modification plays an essential function in regulating gene expression and diverse biological processes. RNA modification enzyme methyltransferase-like 3 (METTL3) affects tumor progression by regulating the N6-methyladenosine (m6A) modification in the mRNAs of critical oncogenes or tumor suppressors, but its effect in oral squamous cell carcinoma (OSCC) remains unknown. In this study, we revealed that METTL3 was consistently upregulated in two OSCC cohorts, and high METTL3 expression was associated with poor prognosis. Functionally, cell proliferation, self-renewal, migration, and invasion ability in vitro and tumor growth and metastasis in vivo were decreased after METTL3 knockdown in OSCC cells. In contrast, the opposite results were obtained after METTL3 overexpression. In addition, the results obtained with the Mettl3 genetically modified mouse model validated the essential role of Mettl3 in chemical-induced oral carcinogenesis. In mechanism, methylated RNA immunoprecipitation sequencing (MeRIP-seq), MeRIP-quantitative real-time PCR, and luciferase reporter and mutagenesis assays identified that METTL3 mediates the m6A modification in the 3' UTR of BMI1 mRNA. METTL3 promotes BMI1 translation in OSCC under the cooperation with m6A reader IGF2BP1. Our findings revealed that METTL3 promotes OSCC proliferation and metastasis through BMI1 m6A methylation, suggesting that the METTL3-m6A-BMI1 axis may serve as a prognostic biomarker or therapeutic target in patients with OSCC.
Keywords: BMI1; METTL3; OSCC; RNA modification.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. - PubMed
-
- Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 2011;11:9–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

