Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury
- PMID: 32621799
- PMCID: PMC7415659
- DOI: 10.1016/j.cell.2020.06.030
Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury
Abstract
Scar tissue size following myocardial infarction is an independent predictor of cardiovascular outcomes, yet little is known about factors regulating scar size. We demonstrate that collagen V, a minor constituent of heart scars, regulates the size of heart scars after ischemic injury. Depletion of collagen V led to a paradoxical increase in post-infarction scar size with worsening of heart function. A systems genetics approach across 100 in-bred strains of mice demonstrated that collagen V is a critical driver of postinjury heart function. We show that collagen V deficiency alters the mechanical properties of scar tissue, and altered reciprocal feedback between matrix and cells induces expression of mechanosensitive integrins that drive fibroblast activation and increase scar size. Cilengitide, an inhibitor of specific integrins, rescues the phenotype of increased post-injury scarring in collagen-V-deficient mice. These observations demonstrate that collagen V regulates scar size in an integrin-dependent manner.
Keywords: Col5a1; cilengitide; collagen V; fibrosis; heart scar; integrins; scar mechanics.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests. Based on this work, patent no: 63/002,828 “Compositions and methods for treating dysregulated wound healing” has been filed and assigned to the Regents of the University of California.
Figures

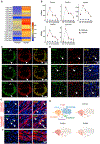


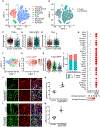


Comment in
-
Type V collagen limits cardiac scar size.Nat Rev Cardiol. 2020 Sep;17(9):539. doi: 10.1038/s41569-020-0423-7. Nat Rev Cardiol. 2020. PMID: 32694670 No abstract available.
References
-
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, and Tamaki K (2005). Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol 175, 7708–7718. - PubMed
-
- Bagnato GL, Irrera N, Pizzino G, Santoro D, Roberts WN, Bagnato G, Pallio G, Vaccaro M, Squadrito F, Saitta A, et al. (2018). Dual alphavbeta3 and alphavbeta5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis. Clin Sci (Lond) 132, 231–242. - PubMed
-
- Barer R (1952). Interference microscopy and mass determination. Nature 169, 366–367. - PubMed
-
- Bashey RI, Martinez-Hernandez A, and Jimenez SA (1992). Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circ Res 70, 1006–1017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL152176/HL/NHLBI NIH HHS/United States
- K99 HL138193/HL/NHLBI NIH HHS/United States
- R00 HL138193/HL/NHLBI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- R01 HL147883/HL/NHLBI NIH HHS/United States
- R01 HL126204/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 HL149658/HL/NHLBI NIH HHS/United States
- R01 AR048179/AR/NIAMS NIH HHS/United States
- T32 AR065972/AR/NIAMS NIH HHS/United States
- R01 HL137241/HL/NHLBI NIH HHS/United States
- R01 HL129178/HL/NHLBI NIH HHS/United States
- R01 HL149687/HL/NHLBI NIH HHS/United States
- R01 AR075867/AR/NIAMS NIH HHS/United States
- K12 GM106996/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

