Intercellular Adhesion Stiffness Moderates Cell Decoupling as a Function of Substrate Stiffness
- PMID: 32621867
- PMCID: PMC7376095
- DOI: 10.1016/j.bpj.2020.05.036
Intercellular Adhesion Stiffness Moderates Cell Decoupling as a Function of Substrate Stiffness
Abstract
The interplay between cell-cell and cell-substrate interactions is complex yet necessary for the formation and healthy functioning of tissues. The same mechanosensing mechanisms used by the cell to sense its extracellular matrix also play a role in intercellular interactions. We used the discrete element method to develop a computational model of a deformable cell that includes subcellular components responsible for mechanosensing. We modeled a three-dimensional cell pair on a patterned (two-dimensional) substrate, a simple laboratory setup to study intercellular interactions. We explicitly modeled focal adhesions and adherens junctions. These mechanosensing adhesions matured, becoming stabilized by force. We also modeled contractile stress fibers that bind the discrete adhesions. The mechanosensing fibers strengthened upon stalling. Traction exerted on the substrate was used to generate traction maps (along the cell-substrate interface). These simulated maps are compared to experimental maps obtained via traction force microscopy. The model recreates the dependence on substrate stiffness of the tractions' spatial distribution, contractile moment of the cell pair, intercellular force, and number of focal adhesions. It also recreates the phenomenon of cell decoupling, in which cells exert forces separately when substrate stiffness increases. More importantly, the model provides viable molecular explanations for decoupling: mechanosensing mechanisms are responsible for competition between different fiber-adhesion configurations present in the cell pair. The point at which an increasing substrate stiffness becomes as high as that of the cell-cell interface is the tipping point at which configurations that favor cell-substrate adhesion dominate over those favoring cell-cell adhesion. This competition is responsible for decoupling.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

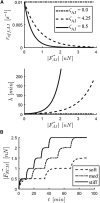
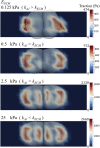
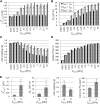

Comment in
-
Stiffness Decouples Cellular Mechanosensation.Biophys J. 2020 Jul 21;119(2):233. doi: 10.1016/j.bpj.2020.06.008. Epub 2020 Jun 12. Biophys J. 2020. PMID: 32610091 Free PMC article. No abstract available.
References
-
- Matsushita T., Oyamada M., Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ. Res. 1999;85:1046–1055. - PubMed
-
- Araujo B.B., Dolhnikoff M., Mauad T. Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur. Respir. J. 2008;32:61–69. - PubMed
-
- Tan C., Costello P., Dedhar S. Inhibition of integrin linked kinase (ILK) suppresses β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC-/- human colon carcinoma cells. Oncogene. 2001;20:133–140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

