Skin sensitization in silico protocol
- PMID: 32621976
- PMCID: PMC7518315
- DOI: 10.1016/j.yrtph.2020.104688
Skin sensitization in silico protocol
Abstract
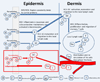
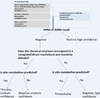
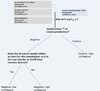
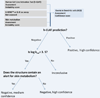
The assessment of skin sensitization has evolved over the past few years to include in vitro assessments of key events along the adverse outcome pathway and opportunistically capitalize on the strengths of in silico methods to support a weight of evidence assessment without conducting a test in animals. While in silico methods vary greatly in their purpose and format; there is a need to standardize the underlying principles on which such models are developed and to make transparent the implications for the uncertainty in the overall assessment. In this contribution, the relationship between skin sensitization relevant effects, mechanisms, and endpoints are built into a hazard assessment framework. Based on the relevance of the mechanisms and effects as well as the strengths and limitations of the experimental systems used to identify them, rules and principles are defined for deriving skin sensitization in silico assessments. Further, the assignments of reliability and confidence scores that reflect the overall strength of the assessment are discussed. This skin sensitization protocol supports the implementation and acceptance of in silico approaches for the prediction of skin sensitization.
Keywords: (Q)SAR; Computational toxicology; Computational toxicology protocols; Defined approach; Expert alerts; Expert review; Extractables and leachables; In silico; In silico toxicology; Integrated approaches to testing and assessment (IATA); Skin sensitization.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




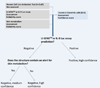

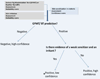




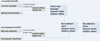
References
-
- Aptula Aynur O. and Roberts David W.. 2006. “Mechanistic Applicability Domains for Nonanimal-Based Prediction of Toxicological End Points: General Principles and Application to Reactive Toxicity.” Chemical Research in Toxicology 19(8):1097–1105. - PubMed
-
- Ball Nicholas, Cagen Stuart, Carrillo Juan-Carlos, Certa Hans, Eigler Dorothea, Emter Roger, Faulhammer Frank, Garcia Christine, Graham Cynthia, Haux Carl, Kolle Susanne N., Kreiling Reinhard, Natsch Andreas, and Mehling Annette. 2011. “Evaluating the Sensitization Potential of Surfactants: Integrating Data from the Local Lymph Node Assay, Guinea Pig Maximization Test, and in Vitro Methods in a Weight-of-Evidence Approach.” Regulatory Toxicology and Pharmacology 60(3):389–400. - PubMed
-
- Basketter DA, Gerberick GF, and Kimber I. 2001. “Skin Sensitisation, Vehicle Effects and the Local Lymph Node Assay.” Food and Chemical Toxicology 39(6):621–27. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

