Immune metabolism in PD-1 blockade-based cancer immunotherapy
- PMID: 32622347
- PMCID: PMC7771015
- DOI: 10.1093/intimm/dxaa046
Immune metabolism in PD-1 blockade-based cancer immunotherapy
Abstract
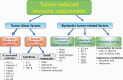
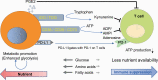
Energy metabolism plays an important role in proliferating cells. Recent reports indicate that metabolic regulation or metabolic products can control immune cell differentiation, fate and reactions. Cancer immunotherapy based on blockade of programmed cell death protein 1 (PD-1) has been used worldwide, but a significant fraction of patients remain unresponsive. Therefore, clarifying the mechanisms and overcoming the unresponsiveness are urgent issues. Because cancer immunity consists of interactions between the cancer and host immune cells, there has recently been a focus on the metabolic interactions and/or competition between the tumor and the immune system to address these issues. Cancer cells render their microenvironment immunosuppressive, driving T-cell dysfunction or exhaustion, which is advantageous for cancer cell survival. However, accumulating mechanistic evidence of T-cell and cancer cell metabolism has gradually revealed that controlling the metabolic pathways of either type of cell can overcome T-cell dysfunction and reprogram the metabolic balance in the tumor microenvironment. Here, we summarize the role of immune metabolism in T-cell-based immune surveillance and cancer immune escape. This new concept has boosted the development of combination therapy and predictive biomarkers in cancer immunotherapy with immune checkpoint inhibitors.
Keywords: biomarker; combination therapy; energy metabolism; immune checkpoint; mitochondria.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Figures


References
-
- Chowdhury, P. S., Chamoto, K. and Honjo, T. 2018. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J. Intern. Med. 283:110. - PubMed
-
- Andrews, L. P., Yano, H. and Vignali, D. A. A. 2019. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 20:1425. - PubMed

