The efficacy and safety of the addition of poly ADP-ribose polymerase (PARP) inhibitors to therapy for ovarian cancer: a systematic review and meta-analysis
- PMID: 32622363
- PMCID: PMC7335450
- DOI: 10.1186/s12957-020-01931-7
The efficacy and safety of the addition of poly ADP-ribose polymerase (PARP) inhibitors to therapy for ovarian cancer: a systematic review and meta-analysis
Abstract
Background: The purpose of this study was to explore the efficacy and tolerability of poly ADP-ribose polymerase (PARP) inhibitors in patients with ovarian cancer.
Methods: The meta-analysis searched the PubMed, Web of Science, EBSCO, and Cochrane libraries from inception to February 2020 to identify relevant studies. And the main results of this study were long-term prognosis and treatment-related adverse events.
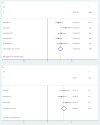
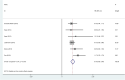
Results: The results showed that the addition of PARP inhibitors could significantly prolong progression-free survival (PFS) and overall survival (OS) for patients with ovarian cancer (HR 0.44, 95% CI 0.34-0.53, p < 0.001; HR, 0.79, 95% CI 0.65-0.94, p < 0.001, respectively). In the BRCA 1/2 mutation patients, the HR of PFS was 0.29 (p < 0.001), and the HR was 0.51 (p < 0.001) in the no BRCA 1/2 mutation patients. The HR of PFS was 0.40 (p < 0.001) in the homologous recombination deficiency (HRD) mutation patients, while the HR was 0.80 (p < 0.001) in the no HRD mutation patients. Moreover, the analysis found that the use of PARP inhibitors did not significantly increase the risk of all grade adverse events (AEs) (RR = 1.04, p = 0.16). But the incidence of grade 3 or higher AEs was increased (RR = 1.87, p = 0.002). In general, the AEs were mainly manifested in the blood system.
Conclusions: PARP inhibitors can improve the prognosis of ovarian cancer patients with and without genetic mutations (BRCA 1/2 or HRD). Furthermore, PARP inhibitors were tolerable to patients when added to their current therapy, although it inevitably adds the grade 3 and higher AEs.
Keywords: BRCA 1/2; HRD; Meta-analysis; Ovarian cancer; PARP inhibitor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27(9):1419–1425. - PMC - PubMed
-
- Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I, Vergote I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Annals Oncol. 2019;30(5):672–705. - PubMed
-
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. The Lancet Oncology. 2014;15(1):23–34. - PubMed
-
- du Bois A, Weber B, Rochon J, Meier W, Goupil A, Olbricht S, Barats JC, Kuhn W, Orfeuvre H, Wagner U, et al. Addition of epirubicin as a third drug to carboplatin-paclitaxel in first-line treatment of advanced ovarian cancer: a prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens. J Clin Oncol. 2006;24(7):1127–1135. - PubMed
-
- Pfisterer J, Weber B, Reuss A, Kimmig R, du Bois A, Wagner U, Bourgeois H, Meier W, Costa S, Blohmer JU, et al. Randomized phase III trial of topotecan following carboplatin and paclitaxel in first-line treatment of advanced ovarian cancer: a gynecologic cancer intergroup trial of the AGO-OVAR and GINECO. Journal of the National Cancer Institute. 2006;98(15):1036–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

