Ambulatory management of primary spontaneous pneumothorax: an open-label, randomised controlled trial
- PMID: 32622394
- PMCID: PMC7607300
- DOI: 10.1016/S0140-6736(20)31043-6
Ambulatory management of primary spontaneous pneumothorax: an open-label, randomised controlled trial
Abstract
Background: Primary spontaneous pneumothorax occurs in otherwise healthy young patients. Optimal management is not defined and often results in prolonged hospitalisation. Data on efficacy of ambulatory options are poor. We aimed to describe the duration of hospitalisation and safety of ambulatory management compared with standard care.
Methods: In this open-label, randomised controlled trial, adults (aged 16-55 years) with symptomatic primary spontaneous pneumothorax were recruited from 24 UK hospitals during a period of 3 years. Patients were randomly assigned (1:1) to treatment with either an ambulatory device or standard guideline-based management (aspiration, standard chest tube insertion, or both). The primary outcome was total length of hospital stay including re-admission up to 30 days after randomisation. Patients with available data were included in the primary analysis and all assigned patients were included in the safety analysis. The trial was prospectively registered with the International Standard Randomised Clinical Trials Number, ISRCTN79151659.
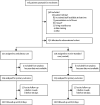
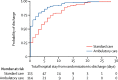
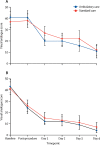
Findings: Of 776 patients screened between July, 2015, and March, 2019, 236 (30%) were randomly assigned to ambulatory care (n=117) and standard care (n=119). At day 30, the median hospitalisation was significantly shorter in the 114 patients with available data who received ambulatory treatment (0 days [IQR 0-3]) than in the 113 with available data who received standard care (4 days [IQR 0-8]; p<0·0001; median difference 2 days [95% CI 1-3]). 110 (47%) of 236 patients had adverse events, including 64 (55%) of 117 patients in the ambulatory care arm and 46 (39%) of 119 in the standard care arm. All 14 serious adverse events occurred in patients who received ambulatory care, eight (57%) of which were related to the intervention, including an enlarging pneumothorax, asymptomatic pulmonary oedema, and the device malfunctioning, leaking, or dislodging.
Interpretation: Ambulatory management of primary spontaneous pneumothorax significantly reduced the duration of hospitalisation including re-admissions in the first 30 days, but at the expense of increased adverse events. This data suggests that primary spontaneous pneumothorax can be managed for outpatients, using ambulatory devices in those who require intervention.
Funding: UK National Institute for Health Research.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Ambulatory management of primary spontaneous pneumothorax: when less is more.Lancet. 2020 Jul 4;396(10243):4-5. doi: 10.1016/S0140-6736(20)31306-4. Lancet. 2020. PMID: 32622395 No abstract available.
-
Management of primary spontaneous pneumothorax: less is more.Lancet. 2021 Dec 19;396(10267):1973. doi: 10.1016/S0140-6736(20)32674-X. Lancet. 2021. PMID: 33341134 No abstract available.
-
Is ambulatory management of primary spontaneous pneumothorax safe and effective?CJEM. 2021 Nov;23(6):750-751. doi: 10.1007/s43678-021-00183-y. Epub 2021 Aug 6. CJEM. 2021. PMID: 34357585 No abstract available.
References
-
- Brown SGA, Ball EL, Perrin K. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405–415. - PubMed
-
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii18–ii31. - PubMed
-
- Schoenenberger RA, Haefeli WE, Weiss P, Ritz RF. Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126:764–766. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

