Thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate for men with lower urinary tract symptoms or urinary retention (UNBLOCS): a randomised controlled trial
- PMID: 32622397
- PMCID: PMC7339133
- DOI: 10.1016/S0140-6736(20)30537-7
Thulium laser transurethral vaporesection of the prostate versus transurethral resection of the prostate for men with lower urinary tract symptoms or urinary retention (UNBLOCS): a randomised controlled trial
Abstract
Background: Transurethral resection of the prostate (TURP) is the standard operation for benign prostatic obstruction. Thulium laser transurethral vaporesection of the prostate (ThuVARP) is a technique with suggested advantages over TURP, including reduced complications and hospital stay. We aimed to investigate TURP versus ThuVARP in men with lower urinary tract symptoms or urinary retention secondary to benign prostatic obstruction.
Methods: In this randomised, blinded, parallel-group, pragmatic equivalence trial, men in seven UK hospitals with bothersome lower urinary tract symptoms or urinary retention secondary to benign prostatic obstruction were randomly assigned (1:1) at the point of surgery to receive ThuVARP or TURP. Patients were masked until follow-up completion. Centres used their usual TURP procedure (monopolar or bipolar). All trial surgeons underwent training on the ThuVARP technique. Co-primary outcomes were maximum urinary flow rate (Qmax) and International Prostate Symptom Score (IPSS) at 12-months post-surgery. Equivalence was defined as a difference of 2·5 points or less for IPSS and 4 mL per s or less for Qmax. Analysis was done according to the intention-to-treat principle. The trial is registered with the ISRCTN Registry, ISRCTN00788389.
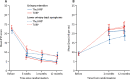
Findings: Between July 23, 2014, and Dec 30, 2016, 410 men were randomly assigned to ThuVARP or TURP, 205 per study group. TURP was superior for Qmax (mean 23·2 mL per s for TURP and 20·2 mL per s for ThuVARP; adjusted difference in means -3·12, 95% CI -5·79 to -0·45). Equivalence was shown for IPSS (mean 6·3 for TURP and 6·4 for ThuVARP; adjusted difference in means 0·28, -0·92 to 1·49). Mean hospital stay was 48 h in both study groups. 91 (45%) of 204 patients in the TURP group and 96 (47%) of 203 patients in the ThuVARP group had at least one complication.
Interpretation: TURP and ThuVARP were equivalent for urinary symptom improvement (IPSS) 12-months post-surgery, and TURP was superior for Qmax. Anticipated laser benefits for ThuVARP of reduced hospital stay and complications were not observed.
Funding: UK National Institute for Health Research Health Technology Assessment Programme.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Surgery for benign prostatic obstruction.Lancet. 2020 Jul 4;396(10243):5-7. doi: 10.1016/S0140-6736(20)31287-3. Lancet. 2020. PMID: 32622396 No abstract available.
-
Re: Thulium Laser Transurethral Vaporesection of the Prostate Versus Transurethral Resection of the Prostate for Men with Lower Urinary Tract Symptoms or Urinary Retention (UNBLOCS): A Randomized Controlled Trial.Eur Urol. 2021 Feb;79(2):317-318. doi: 10.1016/j.eururo.2020.09.011. Epub 2020 Sep 18. Eur Urol. 2021. PMID: 32958299 No abstract available.
-
Re: Thulium Laser Transurethral Vaporesection of the Prostate Versus Transurethral Resection of the Prostate for Men with Lower Urinary Tract Symptoms or Urinary Retention (UNBLOCS): A Randomised Controlled Trial.Eur Urol. 2021 Feb;79(2):316-317. doi: 10.1016/j.eururo.2020.11.035. Epub 2020 Dec 9. Eur Urol. 2021. PMID: 33309032 No abstract available.
References
-
- Hunter DJ, McKee M, Black NA, Sanderson CF. Health status and quality of life of British men with lower urinary tract symptoms: results from the SF-36. Urology. 1995;45:962–971. - PubMed
-
- Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH)—focus on the UK. BJU Int. 2015;115:508–519. - PubMed
-
- Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)—incidence, management, and prevention. Eur Urol. 2006;50:969–979. - PubMed
-
- National Institute for Health and Care Excellence Lower urinary tract symptoms in men: management. May 23, 2010. https://www.nice.org.uk/guidance/cg97 - PubMed
-
- National Institute for Health and Care Excellence GreenLight XPS for treating benign prostatic hyperplasia. June 16, 2016. https://www.nice.org.uk/guidance/mtg29
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

