Conformational Basis of G Protein-Coupled Receptor Signaling Versatility
- PMID: 32622699
- PMCID: PMC7483927
- DOI: 10.1016/j.tcb.2020.06.002
Conformational Basis of G Protein-Coupled Receptor Signaling Versatility
Abstract
G protein-coupled receptors (GPCRs) are privileged structural scaffolds in biology that have the versatility to regulate diverse physiological processes. Interestingly, many GPCR ligands exhibit significant 'bias' - the ability to preferentially activate subsets of the many cellular pathways downstream of these receptors. Recently, complementary information from structural and spectroscopic approaches has made significant inroads into understanding the mechanisms of these biased ligands. The consistently emerging theme is that GPCRs are highly dynamic proteins, and ligands with varying pharmacological properties differentially modulate the equilibrium among multiple conformations. Biased signaling and other recently appreciated complexities of GPCR signaling thus appear to be a natural consequence of the conformational heterogeneity of GPCRs and GPCR-transducer complexes.
Keywords: G protein-coupled receptor; arrestin; biased ligand; biophysics; cellular signaling; pharmacology.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
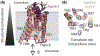


References
-
- Kozielewicz P, et al. (2020) Molecular pharmacology of class F receptor activation. Mol. Pharmacol 97, 62–71 - PubMed
-
- Leach K and Gregory KJ (2017) Molecular insights into allosteric modulation of Class C G protein-coupled receptors. Pharmacol. Res 116, 105–118 - PubMed
-
- Gilman AG (1995) Nobel Lecture. G proteins and regulation of adenylyl cyclase. Biosci. Rep 15, 65–97 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

