Chimeric Antigen Receptor Therapy: How Are We Driving in Solid Tumors?
- PMID: 32623078
- PMCID: PMC11409837
- DOI: 10.1016/j.bbmt.2020.06.020
Chimeric Antigen Receptor Therapy: How Are We Driving in Solid Tumors?
Abstract
Immune effector cell (IEC) therapy is emerging as a promising approach in the field of cancer immunotherapy. Clinical IEC trials, predominantly using chimeric antigen receptor (CAR) T cells, have shown excellent responses in CD19+ B cell malignancies and multiple myeloma. In solid tumors, preclinical data are encouraging, but clinical data are in their infancy, and there are challenges in using CAR T therapy in this setting, including (1) on-target off-tumor toxicity, (2) optimal target identification, (3) effective trafficking into bulky tumor tissue, and (4) resistance to tumor immune evasion mechanisms. Novel techniques and modifications are being explored in both the preclinical and clinical settings, aiming to improve treatment efficacy and address the aforementioned obstacles to successful CAR T therapy in solid tumors. Here we review these challenges in a clinically oriented approach and summarize published clinical trials using CAR T therapy in a variety of solid tumors.
Keywords: Cancer immunotherapy; Chimeric antigen receptor T cells; Immune effector cell therapy; Solid tumor; T cell receptor.
Copyright © 2020 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
E.J.S. serves on the scientific advisory boards of Magenta, Novartis, Celgene, Adaptimmune, and Zelluna. K.R. has a licensing agreement with Takeda; has received educational grants from Affymed and Pharmacyclics; and serves on scientific advisory boards of Virogen, Adicet Bio, Formula Pharma, and GemoAb. G.B. reports grants from Adaptimmune, Elelixis, GlaxoSmithKline, Immatics, Immunocore, Incyte, Kite Pharma, Macrogenics, and Torque; personal fees from Clovis Oncology, Abbvie, Adicet, Amgen, Ariad, Virogin Biotech, Johnson & Johnson/Janssen, and Maverick Therapeutics; and grants and personal fees from Novartis, Bayer, AstraZeneca, Bristol-Myers Squibb, Celgene, Genetech, MedImmune, Merck, Roche, and Xcovery. D.S.H. reports research/grant funding from AbbVie, Adaptimmune, Aldi-Norte, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, GSK, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, MedImmune, Mirati, miRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, Takeda, Turning Point Therapeutics; has received reimbursement for travel, accommodations, and expenses from Bayer, Genmab, AACR, ASCO, SITC. Consulting or Advisory Role: Alpha Insights, Amgen, Axiom, Adaptimmune, Baxter, Bayer, eCancer, Genentech, GLG, Group H, Guidepoint, Infinity, Medscape, Numab, Oncology Education Project Association, Pfizer, Prime Oncology, Takeda, Trieza Therapeutics, and WebMD; and has other ownership interests in Molecular Match (advisor), OncoResponse (founder), and Presagia (advisor). P.K. has received research support from Amgen and Ziopharm; has served on advisory boards for Pfizer, Kite, and Novartis; and has received consulting fees from Jazz Pharmaceuticals.
Figures
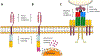

References
-
- Levine BL. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther. 2015;22:79–84. - PubMed
-
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immuneglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

