Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19
- PMID: 32623082
- PMCID: PMC7330574
- DOI: 10.1016/j.ijid.2020.06.099
Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19
Abstract
Significance: The United States is in an acceleration phase of the COVID-19 pandemic. Currently there is no known effective therapy or vaccine for treatment of SARS-CoV-2, highlighting urgency around identifying effective therapies.
Objective: The purpose of this study was to evaluate the role of hydroxychloroquine therapy alone and in combination with azithromycin in hospitalized patients positive for COVID-19.
Design: Multi-center retrospective observational study.
Setting: The Henry Ford Health System (HFHS) in Southeast Michigan: large six hospital integrated health system; the largest of hospitals is an 802-bed quaternary academic teaching hospital in urban Detroit, Michigan.
Participants: Consecutive patients hospitalized with a COVID-related admission in the health system from March 10, 2020 to May 2, 2020 were included. Only the first admission was included for patients with multiple admissions. All patients evaluated were 18 years of age and older and were treated as inpatients for at least 48h unless expired within 24h.
Exposure: Receipt of hydroxychloroquine alone, hydroxychloroquine in combination with azithromycin, azithromycin alone, or neither.
Main outcome: The primary outcome was in-hospital mortality.
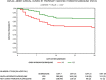
Results: Of 2,541 patients, with a median total hospitalization time of 6 days (IQR: 4-10 days), median age was 64 years (IQR:53-76 years), 51% male, 56% African American, with median time to follow-up of 28.5 days (IQR:3-53). Overall in-hospital mortality was 18.1% (95% CI:16.6%-19.7%); by treatment: hydroxychloroquine+azithromycin, 157/783 (20.1% [95% CI: 17.3%-23.0%]), hydroxychloroquine alone, 162/1202 (13.5% [95% CI: 11.6%-15.5%]), azithromycin alone, 33/147 (22.4% [95% CI: 16.0%-30.1%]), and neither drug, 108/409 (26.4% [95% CI: 22.2%-31.0%]). Primary cause of mortality was respiratory failure (88%); no patient had documented torsades de pointes. From Cox regression modeling, predictors of mortality were age>65 years (HR:2.6 [95% CI:1.9-3.3]), white race (HR:1.7 [95% CI:1.4-2.1]), CKD (HR:1.7 [95%CI:1.4-2.1]), reduced O2 saturation level on admission (HR:1.5 [95%CI:1.1-2.1]), and ventilator use during admission (HR: 2.2 [95%CI:1.4-3.3]). Hydroxychloroquine provided a 66% hazard ratio reduction, and hydroxychloroquine+azithromycin 71% compared to neither treatment (p<0.001).
Conclusions and relevance: In this multi-hospital assessment, when controlling for COVID-19 risk factors, treatment with hydroxychloroquine alone and in combination with azithromycin was associated with reduction in COVID-19 associated mortality. Prospective trials are needed to examine this impact.
Keywords: COVID-19; Coronavirus; Hydroxychloroquine; Mortality; SARS-COV-2; Therapy.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Clarifying the record on hydroxychloroquine for the treatment of patients hospitalized with COVID-19.Int J Infect Dis. 2020 Oct;99:38-39. doi: 10.1016/j.ijid.2020.07.055. Epub 2020 Jul 29. Int J Infect Dis. 2020. PMID: 32738483 Free PMC article. No abstract available.
-
The continued dilemma about the usage of hydroxychloroquine: Respite is in randomized control trials.Int J Infect Dis. 2020 Oct;99:310-311. doi: 10.1016/j.ijid.2020.07.054. Epub 2020 Jul 29. Int J Infect Dis. 2020. PMID: 32738490 Free PMC article. No abstract available.
-
Effectiveness of hydroxychloroquine in COVID-19 disease: A done and dusted deal?Int J Infect Dis. 2020 Oct;99:75-76. doi: 10.1016/j.ijid.2020.07.056. Epub 2020 Jul 29. Int J Infect Dis. 2020. PMID: 32738491 Free PMC article. No abstract available.
-
Problems with the analysis in "Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19".Int J Infect Dis. 2020 Oct;99:37. doi: 10.1016/j.ijid.2020.07.057. Epub 2020 Jul 30. Int J Infect Dis. 2020. PMID: 32738492 Free PMC article. No abstract available.
-
Possible synergistic effects of hydroxychloroquine and steroids in COVID-19, time for a nuanced approach. Comment on Arshad et al.Int J Infect Dis. 2020 Oct;99:344-345. doi: 10.1016/j.ijid.2020.07.064. Epub 2020 Aug 5. Int J Infect Dis. 2020. PMID: 32768694 Free PMC article. No abstract available.
-
Hydroxychloroquine in COVID-19: Taking care of statistics to take care of patients.Int J Infect Dis. 2020 Oct;99:324. doi: 10.1016/j.ijid.2020.07.079. Epub 2020 Aug 6. Int J Infect Dis. 2020. PMID: 32768698 Free PMC article. No abstract available.
-
Comment on Arshad et al.: Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19.Int J Infect Dis. 2020 Oct;99:373. doi: 10.1016/j.ijid.2020.07.071. Epub 2020 Aug 6. Int J Infect Dis. 2020. PMID: 32771630 Free PMC article. No abstract available.
References
-
- CDC . 2020. Cases in the U.S. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Published 27 May 2020. [Accessed 28 May 2020]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous