Peri- and Postpubertal Estrogen Exposures of Female Mice Optimize Uterine Responses Later in Life
- PMID: 32623449
- PMCID: PMC7417879
- DOI: 10.1210/endocr/bqaa081
Peri- and Postpubertal Estrogen Exposures of Female Mice Optimize Uterine Responses Later in Life
Abstract
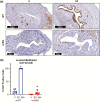
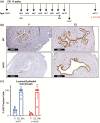
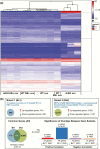
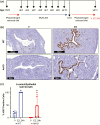
At birth, all female mice, including those that either lack estrogen receptor α (ERα-knockout) or that express mutated forms of ERα (AF2ERKI), have a hypoplastic uterus. However, uterine growth and development that normally accompany pubertal maturation does not occur in ERα-knockout or AF2ERKI mice, indicating ERα-mediated estrogen (E2) signaling is essential for this process. Mice that lack Cyp19 (aromatase knockout, ArKO mice), an enzyme critical for E2 synthesis, are unable to make E2 and lack pubertal uterine development. A single injection of E2 into ovariectomized adult (10 weeks old) females normally results in uterine epithelial cell proliferation; however, we observe that although ERα is present in the ArKO uterine cells, no proliferative response is seen. We assessed the impact of exposing ArKO mice to E2 during pubertal and postpubertal windows and observed that E2-exposed ArKO mice acquired growth responsiveness. Analysis of differential gene expression between unexposed ArKO samples and samples from animals exhibiting the ability to mount an E2-induced uterine growth response (wild-type [WT] or E2-exposed ArKO) revealed activation of enhancer of zeste homolog 2 (EZH2) and heart- and neural crest derivatives-expressed protein 2 (HAND2) signaling and inhibition of GLI Family Zinc Finger 1 (GLI1) responses. EZH2 and HAND2 are known to inhibit uterine growth, and GLI1 is involved in Indian hedgehog signaling, which is a positive mediator of uterine response. Finally, we show that exposure of ArKO females to dietary phytoestrogens results in their acquisition of uterine growth competence. Altogether, our findings suggest that pubertal levels of endogenous and exogenous estrogens impact biological function of uterine cells later in life via ERα-dependent mechanisms.
Keywords: aromatase; estrogen; phytoestrogen; uterine cell growth.
Published by Oxford University Press on behalf of the Endocrine Society 2020.
Figures



Comment in
-
The Window of Vulnerability for Uterus.Endocrinology. 2020 Aug 1;161(8):bqaa104. doi: 10.1210/endocr/bqaa104. Endocrinology. 2020. PMID: 32692842 No abstract available.
References
-
- Klotz DM, Hewitt SC, Ciana P, et al. . Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277(10):8531-8537. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

