Biglycan in the Skeleton
- PMID: 32623936
- PMCID: PMC7649967
- DOI: 10.1369/0022155420937371
Biglycan in the Skeleton
Abstract
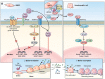
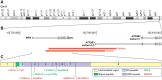
Small leucine rich proteoglycans (SLRPs), including Biglycan, have key roles in many organ and tissue systems. The goal of this article is to review the function of Biglycan and other related SLRPs in mineralizing tissues of the skeleton. The review is divided into sections that include Biglycan's role in structural biology, signaling, craniofacial and long bone homeostasis, remodeled skeletal tissues, and in human genetics. While many cell types in the skeleton are now known to be affected by Biglycan, there are still unanswered questions about its mechanism of action(s).
Keywords: extracellular matrix; glycosaminoglycan; proteoglycan.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

