Cell Energy Metabolism and Hyaluronan Synthesis
- PMID: 32623953
- PMCID: PMC7780193
- DOI: 10.1369/0022155420929772
Cell Energy Metabolism and Hyaluronan Synthesis
Abstract
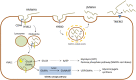
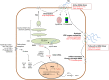
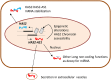
Hyaluronan (HA) is a linear glycosaminoglycan (GAG) of extracellular matrix (ECM) synthesized by three hyaluronan synthases (HASes) at the plasma membrane using uridine diphosphate (UDP)-glucuronic acid (UDP-GlcUA) and UDP-N-acetylglucosamine (UDP-GlcNAc) as substrates. The production of HA is mainly regulated by hyaluronan synthase 2 (HAS2), that can be controlled at different levels, from epigenetics to transcriptional and post-translational modifications. HA biosynthesis is an energy-consuming process and, along with HA catabolism, is strongly connected to the maintenance of metabolic homeostasis. The cytoplasmic pool of UDP-sugars is critical for HA synthesis. UDP-GlcNAc is an important nutrient sensor and serves as donor substrate for the O-GlcNAcylation of many cytosolic proteins, including HAS2. This post-translational modification stabilizes HAS2 in the membrane and increases HA production. Conversely, HAS2 can be phosphorylated by AMP activated protein kinase (AMPK), a master metabolic regulator activated by low ATP/AMP ratios, which inhibits HA secretion. Similarly, HAS2 expression and the deposition of HA within the pericellular coat are inhibited by sirtuin 1 (SIRT1), another important energetic sensor, confirming the tight connection between nutrients availability and HA metabolism.
Keywords: HAS2-AS1; UGDH; autophagy; beta glycosidases; cancer; cardiovascular diseases; extracellular matrix; glycosaminoglycan; hexosamine biosynthetic pathway; hyaluronidase; metabolic reprogramming; proteoglycan; ubiquitin.
Conflict of interest statement
Figures

References
-
- Sainio A, Järveläinen H. Extracellular matrix-cell interactions: focus on therapeutic applications. Cell Signal. 2020;66:109487. - PubMed
-
- Maquart FX, Pasco S, Ramont L, Monboisse J-C. An introduction to matrikines: extracellular matrix—derived peptides which regulate cell activity—implication in tumor invasion. Crit Rev Oncol/Hematol. 2004;49:199–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

