A novel LED-based 2D-fluorescence spectroscopy system for in-line monitoring of Chinese hamster ovary cell cultivations - Part I
- PMID: 32625014
- PMCID: PMC6999564
- DOI: 10.1002/elsc.201800149
A novel LED-based 2D-fluorescence spectroscopy system for in-line monitoring of Chinese hamster ovary cell cultivations - Part I
Abstract
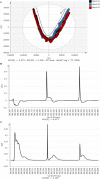
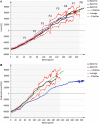
A new two-dimensional fluorescence sensor system was developed for in-line monitoring of mammalian cell cultures. Fluorescence spectroscopy allows for the detection and quantification of naturally occurring intra- and extracellular fluorophores in the cell broth. The fluorescence signals correlate to the cells' current redox state and other relevant process parameters. Cell culture pretests with twelve different excitation wavelengths showed that only three wavelengths account for a vast majority of spectral variation. Accordingly, the newly developed device utilizes three high-power LEDs as excitation sources in combination with a back-thinned CCD-spectrometer for fluorescence detection. This setup was first tested in a lab design of experiments study with process relevant fluorophores proving its suitability for cell culture monitoring with LOD in the μg/L range. The sensor was then integrated into a CHO-K1 cell culture process. The acquired fluorescence spectra of several batches were evaluated using multivariate methods. The resulting batch evolution models were challenged in deviating and "golden batch" validation runs. These first tests showed that the new sensor can trace the cells' metabolic state in a fast and reliable manner. Cellular distress is quickly detected as a deviation from the "golden batch".
Keywords: 2D‐fluorescence spectroscopy; CHO cell cultivation; MVDA; PAT; in‐line bioprocess monitoring; metabolism monitoring.
© 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures





References
-
- Classen, J. , Aupert, F. , Reardon, K. F. , Solle, D. et al., Spectroscopic sensors for in‐line bioprocess monitoring in research and pharmaceutical industrial application. Anal. Bioanal. Chem. 2017, 409, 651–666. - PubMed
-
- Bakeev K. A. (Ed.), Process Analytical Technology: Spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries, 2nd Ed, John Wiley & Sons, Ltd, Chichester, UK: 2010.
-
- Dickens, J. E. , Fluorescent Sensing and Process Analytical Applications, in: Bakeev K. A. (Ed.), Process Analytical Technology: Spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries, 2nd Ed, John Wiley & Sons, Ltd, Chichester, UK: 2010, pp. 337–352.
LinkOut - more resources
Full Text Sources
Miscellaneous
