Lignin valorization meets synthetic biology
- PMID: 32625023
- PMCID: PMC6999416
- DOI: 10.1002/elsc.201800133
Lignin valorization meets synthetic biology
Abstract
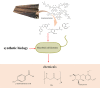
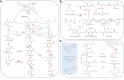
Lignin, an abundant renewable resource in nature, is a highly heterogeneous biopolymer consisting of phenylpropanoid units. It is essential for sustainable utilization of biomass to convert lignin to value-added products. However, there are technical obstacles for lignin valorization due to intrinsic heterogeneity. The emerging of synthetic biology technologies brings new opportunities for lignin breakdown and utilization. In this review, we discussed the applications of synthetic biology on lignin conversion, especially the production of value-added products, such as aromatic chemicals, ring-cleaved chemicals from lignin-derived aromatics and bio-active substances. Synthetic biology will offer new potential strategies for lignin valorization by optimizing lignin degradation enzymes, building novel artificial converting pathways, and improving the chassis of model microorganisms.
Keywords: artificial pathway; bio‐degradation; lignin valorization; synthetic biology; value‐added products.
© 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures
References
-
- Ragauskas, A. J. , Beckham, G. T. , Biddy, M. J. , Chandra, R. et al., Lignin valorization: improving lignin processing in the biorefinery. Science 2014, 344, 1246843. - PubMed
-
- Ralph, J. , Hydroxycinnamates in lignification. Phytochem. Rev. 2009, 9, 65–83.
-
- Sainsbury, P. D. , Hardiman, E. M. , Ahmad, M. , Otani, H. et al., Breaking down lignin to high‐value chemicals: the conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem. Biol. 2013, 8, 2151–2156. - PubMed
-
- Tuck, C. O. , Perez, E. , Horvath, I. T. , Sheldon, R. A. , Poliakoff, M. , Valorization of biomass: deriving more value from waste. Science 2012, 337, 695–699. - PubMed
-
- Zakzeski, J. , Bruijnincx, P. C. A. , Jongerius, A. L. , Weckhuysen, B. M. , The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
