LRRK2 and α-Synuclein: Distinct or Synergistic Players in Parkinson's Disease?
- PMID: 32625052
- PMCID: PMC7311858
- DOI: 10.3389/fnins.2020.00577
LRRK2 and α-Synuclein: Distinct or Synergistic Players in Parkinson's Disease?
Abstract
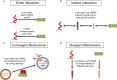
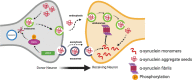
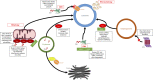
Parkinson's disease (PD) is the most common neurodegenerative movement disorder, characterized by prominent degeneration of dopaminergic neurons in the substantia nigra and aggregation of the protein α-synuclein within intraneuronal inclusions known as Lewy bodies. Ninety percent of PD cases are idiopathic while the remaining 10% are associated with gene mutations that affect cellular functions ranging from kinase activity to mitochondrial quality control, hinting at a multifactorial disease process. Mutations in LRRK2 and SNCA (the gene coding for α-synuclein) cause monogenic forms of autosomal dominant PD, and polymorphisms in either gene are also associated with increased risk of idiopathic PD. Although Lewy bodies are a defining neuropathological feature of PD, an appreciable subset of patients with LRRK2 mutations present with a clinical phenotype indistinguishable from idiopathic PD but lack Lewy pathology at autopsy, suggesting that LRRK2-mediated PD may occur independently of α-synuclein aggregation. Here, we examine whether LRRK2 and α-synuclein, as mediators of neurodegeneration in PD, exist in common or distinct pathways. Specifically, we review evidence from preclinical models and human neuropathological studies examining interactions between the two proteins. Elucidating the degree of interplay between LRRK2 and α-synuclein will be necessary for treatment stratification once effective targeted disease-modifying therapies are developed.
Keywords: LRRK2; Parkinson’s disease; autophagy; mitochondria; neurodegeneration; α-synuclein.
Copyright © 2020 O’Hara, Pawar, Kalia and Kalia.
Figures
References
-
- Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., et al. (2006). Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281 29739–29752. 10.1074/jbc.m600933200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

