Visualizing the Central Nervous System: Imaging Tools for Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders
- PMID: 32625158
- PMCID: PMC7311777
- DOI: 10.3389/fneur.2020.00450
Visualizing the Central Nervous System: Imaging Tools for Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders
Abstract
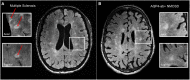
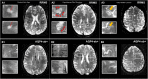
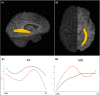
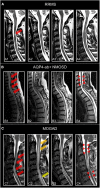
Multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD) are autoimmune central nervous system conditions with increasing incidence and prevalence. While MS is the most frequent inflammatory CNS disorder in young adults, NMOSD is a rare disease, that is pathogenetically distinct from MS, and accounts for approximately 1% of demyelinating disorders, with the relative proportion within the demyelinating CNS diseases varying widely among different races and regions. Most immunomodulatory drugs used in MS are inefficacious or even harmful in NMOSD, emphasizing the need for a timely and accurate diagnosis and distinction from MS. Despite distinct immunopathology and differences in disease course and severity there might be considerable overlap in clinical and imaging findings, posing a diagnostic challenge for managing neurologists. Differential diagnosis is facilitated by positive serology for AQP4-antibodies (AQP4-ab) in NMOSD, but might be difficult in seronegative cases. Imaging of the brain, optic nerve, retina and spinal cord is of paramount importance when managing patients with autoimmune CNS conditions. Once a diagnosis has been established, imaging techniques are often deployed at regular intervals over the disease course as surrogate measures for disease activity and progression and to surveil treatment effects. While the application of some imaging modalities for monitoring of disease course was established decades ago in MS, the situation is unclear in NMOSD where work on longitudinal imaging findings and their association with clinical disability is scant. Moreover, as long-term disability is mostly attack-related in NMOSD and does not stem from insidious progression as in MS, regular follow-up imaging might not be useful in the absence of clinical events. However, with accumulating evidence for covert tissue alteration in NMOSD and with the advent of approved immunotherapies the role of imaging in the management of NMOSD may be reconsidered. By contrast, MS management still faces the challenge of implementing imaging techniques that are capable of monitoring progressive tissue loss in clinical trials and cohort studies into treatment algorithms for individual patients. This article reviews the current status of imaging research in MS and NMOSD with an emphasis on emerging modalities that have the potential to be implemented in clinical practice.
Keywords: magnetic resonance imaging; multiple sclerosis; neuroimaging; neuromyelitis optica spectrum disorders (NMOSD); optical coherence tomography.
Copyright © 2020 Kuchling and Paul.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

