Surface Protein Dispersin of Enteroaggregative Escherichia coli Binds Plasminogen That Is Converted Into Active Plasmin
- PMID: 32625178
- PMCID: PMC7315649
- DOI: 10.3389/fmicb.2020.01222
Surface Protein Dispersin of Enteroaggregative Escherichia coli Binds Plasminogen That Is Converted Into Active Plasmin
Abstract
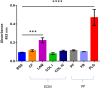
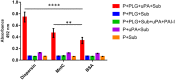
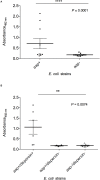
Dispersin is a 10.2 kDa-immunogenic protein secreted by enteroaggregative Escherichia coli (EAEC). In the prototypical EAEC strain 042, dispersin is non-covalently bound to the outer membrane, assisting dispersion across the intestinal mucosa by overcoming electrostatic attraction between the AAF/II fimbriae and the bacterial surface. Also, dispersin facilitates penetration of the intestinal mucus layer. Initially characterized in EAEC, dispersin has been detected in other E. coli pathotypes, including those isolated from extraintestinal sites. In this study we investigated the binding capacity of purified dispersin to extracellular matrix (ECM), since dispersin is exposed on the bacterial surface and is involved in intestinal colonization. Binding to plasminogen was also investigated due to the presence of conserved carboxy-terminal lysine residues in dispersin sequences, which are involved in plasminogen binding in several bacterial proteins. Moreover, some E. coli components can interact with this host protease, as well as with tissue plasminogen activator, leading to plasmin production. Recombinant dispersin was produced and used in binding assays with ECM molecules and coagulation cascade compounds. Purified dispersin bound specifically to laminin and plasminogen. Interaction with plasminogen occurred in a dose-dependent and saturable manner. In the presence of plasminogen activator, bound plasminogen was converted into plasmin, its active form, leading to fibrinogen and vitronectin cleavage. A collection of E. coli strains isolated from human bacteremia was screened for the presence of aap, the dispersin-encoding gene. Eight aap-positive strains were detected and dispersin production could be observed in four of them. Our data describe new attributes for dispersin and points out to possible roles in mechanisms of tissue adhesion and dissemination, considering the binding capacity to laminin, and the generation of dispersin-bound plasmin(ogen), which may facilitate E. coli spread from the colonization site to other tissues and organs. The cleavage of fibrinogen in the bloodstream, may also contribute to the pathogenesis of sepsis caused by dispersin-producing E. coli.
Keywords: EAEC; ECM; dispersin; plasmin; plasminogen.
Copyright © 2020 Moraes, Longo, Silva, Pimenta, Carvalho, Morone, da Rós, Serrano, Santos, Piazza, Barbosa and Elias.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

