Respiratory Immunization With a Whole Cell Inactivated Vaccine Induces Functional Mucosal Immunoglobulins Against Tuberculosis in Mice and Non-human Primates
- PMID: 32625195
- PMCID: PMC7315045
- DOI: 10.3389/fmicb.2020.01339
Respiratory Immunization With a Whole Cell Inactivated Vaccine Induces Functional Mucosal Immunoglobulins Against Tuberculosis in Mice and Non-human Primates
Abstract
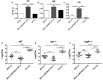
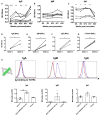
Vaccination through the natural route of infection represents an attractive immunization strategy in vaccinology. In the case of tuberculosis, vaccine delivery by the respiratory route has regained interest in recent years, showing efficacy in different animal models. In this context, respiratory vaccination triggers lung immunological mechanisms which are omitted when vaccines are administered by parenteral route. However, contribution of mucosal antibodies to vaccine- induced protection has been poorly studied. In the present study, we evaluated in mice and non-human primates (NHP) a novel whole cell inactivated vaccine (MTBVAC HK), by mucosal administration. MTBVAC HK given by intranasal route to BCG-primed mice substantially improved the protective efficacy conferred by subcutaneous BCG only. Interestingly, this improved protection was absent in mice lacking polymeric Ig receptor (pIgR), suggesting a crucial role of mucosal secretory immunoglobulins in protective immunity. Our study in NHP confirmed the ability of MTBVAC HK to trigger mucosal immunoglobulins. Importantly, in vitro assays demonstrated the functionality of these immunoglobulins to induce M. tuberculosis opsonization in the presence of human macrophages. Altogether, our results suggest that mucosal immunoglobulins can be induced by vaccination to improve protection against tuberculosis and therefore, they represent a promising target for next generation tuberculosis vaccines.
Keywords: animal models; mucosal immunoglobulins; opsonization; pulmonary vaccination; tuberculosis; whole-cell vaccine.
Copyright © 2020 Aguilo, Uranga, Mata, Tarancon, Gómez, Marinova, Otal, Monzón, Badiola, Montenegro, Puentes, Rodríguez, Vervenne, Sombroek, Verreck and Martín.
Figures




References
-
- Aguilo N., Alvarez-Arguedas S., Uranga S., Marinova D., Monzon M., Badiola J., et al. (2016). Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17-Dependent mechanism. J. Infect. Dis. 213 831–839. 10.1093/infdis/jiv503 - DOI - PubMed
-
- Arbues A., Aguilo J. I., Gonzalo-Asensio J., Marinova D., Uranga S., Puentes E., et al. (2013). Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine 31 4867–4873. 10.1016/j.vaccine.2013.07.051 - DOI - PubMed
-
- Baqui A. A., Meiller T. F., Kelley J. I., Turng B. F., Falkler W. A. (1999). Antigen activation of THP-1 human monocytic cells after stimulation with lipopolysaccharide from oral microorganisms and granulocyte-macrophage colony-stimulating factor. J. Periodontal Res. 34 203–213. 10.1111/j.1600-0765.1999.tb02243.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

