EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA)
- PMID: 32625259
- PMCID: PMC7010070
- DOI: 10.2903/j.efsa.2017.4666
EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA)
Abstract
EFSA and EMA have jointly reviewed measures taken in the EU to reduce the need for and use of antimicrobials in food-producing animals, and the resultant impacts on antimicrobial resistance (AMR). Reduction strategies have been implemented successfully in some Member States. Such strategies include national reduction targets, benchmarking of antimicrobial use, controls on prescribing and restrictions on use of specific critically important antimicrobials, together with improvements to animal husbandry and disease prevention and control measures. Due to the multiplicity of factors contributing to AMR, the impact of any single measure is difficult to quantify, although there is evidence of an association between reduction in antimicrobial use and reduced AMR. To minimise antimicrobial use, a multifaceted integrated approach should be implemented, adapted to local circumstances. Recommended options (non-prioritised) include: development of national strategies; harmonised systems for monitoring antimicrobial use and AMR development; establishing national targets for antimicrobial use reduction; use of on-farm health plans; increasing the responsibility of veterinarians for antimicrobial prescribing; training, education and raising public awareness; increasing the availability of rapid and reliable diagnostics; improving husbandry and management procedures for disease prevention and control; rethinking livestock production systems to reduce inherent disease risk. A limited number of studies provide robust evidence of alternatives to antimicrobials that positively influence health parameters. Possible alternatives include probiotics and prebiotics, competitive exclusion, bacteriophages, immunomodulators, organic acids and teat sealants. Development of a legislative framework that permits the use of specific products as alternatives should be considered. Further research to evaluate the potential of alternative farming systems on reducing AMR is also recommended. Animals suffering from bacterial infections should only be treated with antimicrobials based on veterinary diagnosis and prescription. Options should be reviewed to phase out most preventive use of antimicrobials and to reduce and refine metaphylaxis by applying recognised alternative measures.
Keywords: alternatives; antimicrobial consumption; antimicrobial resistance; control options; husbandry.
© 2017 European Medicines Agency and © European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
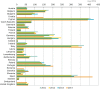
Correction of sales data and/or
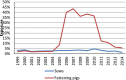
PCU data published inESVAC 2013 report is described in section 1.6 (EMA ESVAC, 2015b). Under‐reported for Bulgaria for 2011 and 2012 as several wholesalers failed to report data. Strength reported as base for mostVMP s for 2011‐2012 for the Czech Republic; for 2013 and 2014, strength reported as in the label of theVMP s. Strength reported as base for someVMP s for 2011–2012 for the Netherlands; for 2013 and 2014, strength reported as in the label of theVMP s. For Slovakia, for 2011 and 2012, the data only represents antimicrobialVMP s imported by wholesalers; for 2013 and 2014, data represents all sales from wholesalers to end users (veterinarians, pharmacies, producers of medicated feeding stuffs and farmers, obtained by import and from national manufacturers). For Spain, under‐reporting for the years 2011–2013 has been identified (underestimated). For theUK , high sales of certain tetracycline‐containing products late in 2010 were probably used in 2011 and thus the use has been underestimated for 2011.
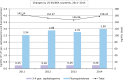
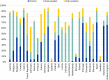

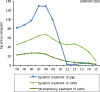


References
-
- Aarestrup FM, 1999. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. International Journal of Antimicrobial Agents, 12, 279–285. - PubMed
-
- Aarestrup FM, 2000. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS, Acta Pathologica, Microbiologica et Immunologica Scandinavica, 108, 0–48. - PubMed
-
- Aarestrup FM, Oliver Duran C and Burch DG, 2008. Antimicrobial resistance in swine production. Animal Health Research Reviews, 9, 135–148. - PubMed
-
- Aarestrup FM, Jensen VF, Emborg H‐D, Jacobsen E and Wegener HC, 2010. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. American Journal of Veterinary Research, 71, 726–733. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
