Re-evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives
- PMID: 32625282
- PMCID: PMC7009865
- DOI: 10.2903/j.efsa.2017.4911
Re-evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives
Abstract
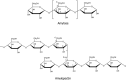
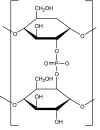
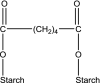
Following a request from the European Commission, the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS) was asked to deliver a scientific opinion on the re-evaluation of 12 modified starches (E 1404, E 1410, E 1412, E 1413, E 1414, E 1420, E 1422, E 1440, E 1442, E 1450, E 1451 and E 1452) authorised as food additives in the EU in accordance with Regulation (EC) No 1333/2008 and previously evaluated by JECFA and the SCF. Both committees allocated an acceptable daily intake (ADI) 'not specified'. In humans, modified starches are not absorbed intact but significantly hydrolysed by intestinal enzymes and then fermented by the intestinal microbiota. Using the read-across approach, the Panel considered that adequate data on short- and long-term toxicity and carcinogenicity, and reproductive toxicity are available. Based on in silico analyses, modified starches are considered not to be of genotoxic concern. No treatment-related effects relevant for human risk assessment were observed in rats fed very high levels of modified starches (up to 31,000 mg/kg body weight (bw) per day). Modified starches (e.g. E 1450) were well tolerated in humans up to a single dose of 25,000 mg/person. Following the conceptual framework for the risk assessment of certain food additives, the Panel concluded that there is no safety concern for the use of modified starches as food additives at the reported uses and use levels for the general population and that there is no need for a numerical ADI. The combined exposure to E 1404-E 1451 at the 95th percentile of the refined (brand-loyal) exposure assessment scenario for the general population was up to 3,053 mg/kg bw per day. Exposure to E 1452 for food supplement consumers only at the 95th percentile was up to 22.1 mg/kg bw per day.
Keywords: E 1404; E 1410; E 1412; E 1413; E 1414; E 1420; E 1422; E 1440; E 1442; E 1450; E 1451; E 1452; modified starches.
© 2017 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
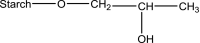


References
-
- Anderson TA, Filer LJ Jr, Fomon SJ, Andersen DW, Jensen RL and Rogers RR, 1973a. Effect of waxy corn starch modification on growth, serum biochemical values and body composition of Pitman−Moore miniature pigs. Food and Cosmetics Toxicology, 11, 747–754. - PubMed
-
- Anderson TA, Fomon SJ and Andersen DW, 1973b. Unpublished data submitted to Corn Refiners Association, Inc., In: Cited in JECFA, 1982e.
-
- Annisson G, Illman RJ and Topping DL, 2003. Acetylated, propionylated or butyrylated starches raise large bowel short‐chain fatty acids preferentially when fed to rats. The Journal of Nutrition, 133, 3523–3528. - PubMed
-
- Anonymous , 1960. Caloric evaluation of RX12XI and cornstarch. Unpublished report of Food Research Laboratories, Inc. (Report No. 80878b‐e). Submitted to the World Health Organization by the National Starch and Chemical Corporation, Bridgewater, NJ, USA. Cited in JECFA, 1982g.
LinkOut - more resources
Full Text Sources
