Avian influenza
- PMID: 32625288
- PMCID: PMC7009867
- DOI: 10.2903/j.efsa.2017.4991
Avian influenza
Abstract
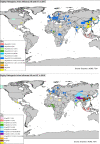
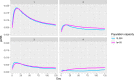
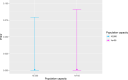
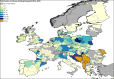
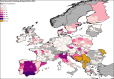
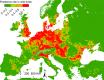
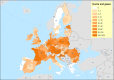
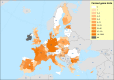
Previous introductions of highly pathogenic avian influenza virus (HPAIV) to the EU were most likely via migratory wild birds. A mathematical model has been developed which indicated that virus amplification and spread may take place when wild bird populations of sufficient size within EU become infected. Low pathogenic avian influenza virus (LPAIV) may reach similar maximum prevalence levels in wild bird populations to HPAIV but the risk of LPAIV infection of a poultry holding was estimated to be lower than that of HPAIV. Only few non-wild bird pathways were identified having a non-negligible risk of AI introduction. The transmission rate between animals within a flock is assessed to be higher for HPAIV than LPAIV. In very few cases, it could be proven that HPAI outbreaks were caused by intrinsic mutation of LPAIV to HPAIV but current knowledge does not allow a prediction as to if, and when this could occur. In gallinaceous poultry, passive surveillance through notification of suspicious clinical signs/mortality was identified as the most effective method for early detection of HPAI outbreaks. For effective surveillance in anseriform poultry, passive surveillance through notification of suspicious clinical signs/mortality needs to be accompanied by serological surveillance and/or a virological surveillance programme of birds found dead (bucket sampling). Serosurveillance is unfit for early warning of LPAI outbreaks at the individual holding level but could be effective in tracing clusters of LPAIV-infected holdings. In wild birds, passive surveillance is an appropriate method for HPAIV surveillance if the HPAIV infections are associated with mortality whereas active wild bird surveillance has a very low efficiency for detecting HPAIV. Experts estimated and emphasised the effect of implementing specific biosecurity measures on reducing the probability of AIV entering into a poultry holding. Human diligence is pivotal to select, implement and maintain specific, effective biosecurity measures.
Keywords: avian influenza; biosecurity; introduction; mutagenesis; spread; surveillance; zoning.
© 2017 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures

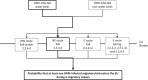
Bold lines, quantitative assessment; thin lines, qualitative assessment.
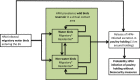
The white fields represent components of the model based on real field situations, whereas the green fields represent components based on theoretical scenarios.
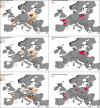
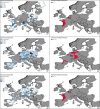
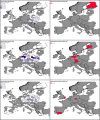
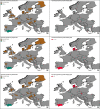
*Four scenarios are considered with respect to proportion of migratory/resident wild birds and proportion of water/non‐water birds (see Table 5).
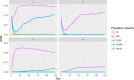

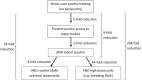

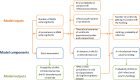

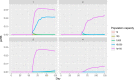
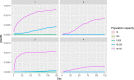
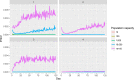
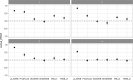
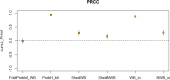
pi_MWB, prevalence of migratory infected water birds; ProbH_b0, probability that a poultry holding is infected due to the presence of infected wild birds; ShedWB, shedding period for water birds; ShedNWB, shedding period for non‐water birds; WG_in, number of water birds present in the holding premise; NWB_in, number of non‐water birds present in the holding premise.






References
-
- Abdelwhab EM, Grund C, Aly MM, Beer M, Harder TC and Hafez HM, 2012. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Veterinary Microbiology, 155, 13–20. - PubMed
-
- Abdelwhab EM, Hassan MK, Abdel‐Moneim AS, Naguib MM, Mostafa A, Hussein ITM, Arafa A, Erfan AM, Kilany WH, Agour MG, El‐Kanawati Z, Husseini HA, Selim AA, Kholousy S, El‐Naggar H, El‐Zoghby EF, Samy A, Iqbal M, Eid A, Ibraheem EM, Pleschka S, Veits J, Nasef SA, Beer M, Mettenleiter TC, Grund C, Ali MM, Harder TC and Hafez HM, 2016. Introduction and enzootic of A/H5N1 in Egypt: virus evolution, pathogenicity and vaccine efficacy ten years on. Infection Genetics and Evolution, 40, 80–90. - PubMed
LinkOut - more resources
Full Text Sources
