Vector-borne diseases
- PMID: 32625493
- PMCID: PMC7009857
- DOI: 10.2903/j.efsa.2017.4793
Vector-borne diseases
Abstract
After a request from the European Commission, EFSA's Panel on Animal Health and Welfare summarised the main characteristics of 36 vector-borne diseases (VBDs) in https://efsa.maps.arcgis.com/apps/PublicGallery/index.html?appid=dfbeac92aea944599ed1eb754aa5e6d1. The risk of introduction in the EU through movement of livestock or pets was assessed for each of the 36 VBDs individually, using a semiquantitative Method to INTegrate all relevant RISK aspects (MINTRISK model), which was further modified to a European scale into the http://www3.lei.wur.nl/mintrisk/ModelMgt.aspx. Only eight of the 36 VBD-agents had an overall rate of introduction in the EU (being the combination of the rate of entry, vector transmission and establishment) which was estimated to be above 0.001 introductions per year. These were Crimean-Congo haemorrhagic fever virus, bluetongue virus, West Nile virus, Schmallenberg virus, Hepatozoon canis, Leishmania infantum, Bunyamwera virus and Highlands J. virus. For these eight diseases, the annual extent of spread was assessed, assuming the implementation of available, authorised prevention and control measures in the EU. Further, the probability of overwintering was assessed, as well as the possible impact of the VBDs on public health, animal health and farm production. For the other 28 VBD-agents for which the rate of introduction was estimated to be very low, no further assessments were made. Due to the uncertainty related to some parameters used for the risk assessment or the instable or unpredictability disease situation in some of the source regions, it is recommended to update the assessment when new information becomes available. Since this risk assessment was carried out for large regions in the EU for many VBD-agents, it should be considered as a first screening. If a more detailed risk assessment for a specific VBD is wished for on a national or subnational level, the EFSA-VBD-RISK-model is freely available for this purpose.
Keywords: vector‐borne diseases.
© 2017 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
*
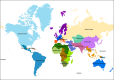
UN ‐regions: see Section 2.3 .
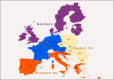



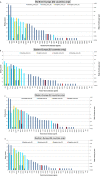
(a): Entry_scores: very low rate of entry: 0–0.2; low rate of entry: 0.2–0.4; moderate rate of entry: 0.4–0.6; high rate of entry: 0.6–0.8; very high rate of entry > 0.8. (b): The entry score (sc) translates into rate of entry (Entry) using the following formula Rate of entry = 10^[5 × (sc − 1.0)]. Thus, an entry score of 1 translates to once per year and 0.8 translates to once per 10 years, 0.6 means once in 100 years, etc. (c):
AHSV : African horse sickness virus;ASFV : African swine fever virus;AINOV : Aino virus;AKAV : Akabane virus;AHFV : Alkhurma haemorrhagic fever virus;BHAV : Bhanja virus;BTV : Bluetongue virus;BEFV : Bovine ephemeral fever virus;CVV : Bunyamwera virus;CCHF : Crimean‐Congo haemorrhagic fever virus;EEE : Eastern equine encephalitis virus;EHDV : Epizootic haemorrhagic disease virus;EEV : Equine encephalosis virus;GETV : Getah virus; Cowdr: Heartwater (Cowdriosis); Hepat: Hepatozoonis; (H. canis);HJV : Highlands J. virus;JEV : Japanese encephalitis virus;KOTV : Kotonkan virus; CanL: Leishmaniosis (L. infantum);MDV : Main Drain virus;MIDV : Middelburg virus;NSDV : Nairobi sheep disease virus;KASV : Palyam virus;PHSV : Peruvian horse sickness virus;RVF : Rift Valley fever virus;SLEV : Saint Louis encephalitis virus;SBV : Schmallenberg virus;SHUV : Shuni virus;THOV : Thogoto virus;VEE : Venezuelan equine encephalitis virus;VSV : Vesicular stomatitis virus;WSLV : Wesselsbron virus;WNV : West Nile fever virus;WEEV : Western equine encephalitis virus;YUOV : Yunnan orbivirus virus.

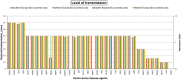
Scores: very low level of transmission: 0–0.2; low level of transmission: 0.2–0.4; moderate level of transmission: 0.4–0.6, high level of transmission: 0.6–0.8, very high level of transmission > 0.8. The transmission score is a measure representing a logarithmically adjusted reproduction ratio, where 0.4 represents a reproduction ratio around 1 (i.e. no epidemic development expected below 0.4. whereas 0.8 represents a reproduction ratio around 10). The transmission score (sc) translates into reproduction ratio (R0) using the following formula: R0 = 10^[2.5 × (sc − 0.4)].
AHSV : African horse sickness virus;ASFV : African swine fever virus;AINOV : Aino virus;AKAV : Akabane virus;AHFV : Alkhurma haemorrhagic fever virus;BHAV : Bhanja virus;BTV : Bluetongue virus;BEFV : Bovine ephemeral fever virus;CVV : Bunyamwera virus;CCHFV : Crimean‐Congo haemorrhagic fever virus;EEE : Eastern equine encephalitis virus;EHDV : Epizootic haemorrhagic disease virus;EEV : Equine encephalosis virus;GETV : Getah virus; Cowdr: Heartwater (Cowdriosis); Hepat: Hepatozoonis; (H. canis);HJV : Highlands J. virus;JEV : Japanese encephalitis virus;KOTV : Kotonkan virus; CanL: Leishmaniosis (L. infantum);MDV : Main Drain virus;MIDV : Middelburg virus;NSDV : Nairobi sheep disease virus;KASV : Palyam virus;PHSV : Peruvian horse sickness virus;RVF : Rift Valley fever virus;SLEV : Saint Louis encephalitis virus;SBV : Schmallenberg virus;SHUV : Shuni virus;THOV : Thogoto virus;VEE : Venezuelan equine encephalitis virus;VSV : Vesicular stomatitis virus;WSLV : Wesselsbron virus;WNV : West Nile fever virus;WEEV : Western equine encephalitis virus;YUOV : Yunnan orbivirus virus.

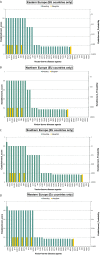
(a): Establishment scores: very low probability of establishment: 0–0.19; low probability of establishment: 0.2–0.39; moderate probability of establishment: 0.4–0.59, high probability of establishment: 0.6–0.79, very high probability of establishment: ≥ 0.8. (b): The establishment score represents the log transformed probability of establishment, where 1 represents certain establishment, 0.8 translates to a probability of 10%, 0.6 translates to a probability of 1%, etc. The establishment score (sc) translates into establishment probability using the following formula: Establishment_Probability = 10^[5 × (sc − 1)]. (c):
AHSV : African horse sickness virus;ASFV : African swine fever virus;AINOV : Aino virus;AKAV : Akabane virus;AHFV : Alkhurma haemorrhagic fever virus;BHAV : Bhanja virus;BTV : Bluetongue virus;BEFV : Bovine ephemeral fever virus;CVV : Bunyamwera virus;CCHF : Crimean‐Congo haemorrhagic fever virus;EEE : Eastern equine encephalitis virus;EHDV : Epizootic haemorrhagic disease virus;EEV : Equine encephalosis virus;GETV : Getah virus; Cowdr: Heartwater (Cowdriosis); Hepat: Hepatozoonis; (H. canis);HJV : Highlands J. virus;JEV : Japanese encephalitis virus;KOTV : Kotonkan virus; CanL: Leishmaniosis (L. infantum);MDV : Main Drain virus;MIDV : Middelburg virus;NSDV : Nairobi sheep disease virus;KASV : Palyam virus;PHSV : Peruvian horse sickness virus;RVF : Rift Valley fever virus;SLEV : Saint Louis encephalitis virus;SBV : Schmallenberg virus;SHUV : Shuni virus;THOV : Thogoto virus;VEE : Venezuelan equine encephalitis virus;VSV : Vesicular stomatitis virus;WSLV : Wesselsbron virus;WNV : West Nile fever virus;WEEV : Western equine encephalitis virus;YUOV : Yunnan orbivirus virus.
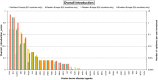
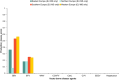
Risk scores: very low rate of introduction: 0–0.2; low rate of introduction: 0.2–0.4; moderate rate of introduction: 0.4–0.6, high rate of introduction: 0.6–0.8, very high rate of introduction: > 0.8. The overall introduction score is a logarithmic translation of the rate of introduction of new epidemics. A score of 1 translates to 10 epidemics starting each year, a score of 0.8 translates to one epidemic per year, 0.6 translates to 1 epidemic every 10 years, etc. The overall introduction score (sc) translates into the number of new epidemics/year (No. epidemics/year) using the following formula: No. epidemics/year = 10^[5 × (sc − 0.8)].
AHSV : African horse sickness virus;ASFV : African swine fever virus;AINOV : Aino virus;AKAV : Akabane virus;AHFV : Alkhurma haemorrhagic fever virus;BHAV : Bhanja virus;BTV : Bluetongue virus;BEFV : Bovine ephemeral fever virus;CVV : Bunyamwera virus;CCHFV : Crimean Congo haemorrhagic fever virus;EEEV : Eastern equine encephalitis virus;EHDV : Epizootic haemorrhagic disease virus;EEV : Equine encephalosis virus;GETV : Getah virus; Cowdr: Heartwater (Cowdriosis); Hepat: Hepatozoonis; (H. canis);HJV : Highlands J. virus;JEV : Japanese encephalitis virus;KOTV : Kotonkan virus; CanL: Leishmaniosis (L. infantum);MDV : Main Drain virus;MIDV : Middelburg virus;NSDV : Nairobi sheep disease virus;KASV : Palyam virus;PHSV : Peruvian horse sickness virus;RVFV : Rift Valley fever virus;SLEV : Saint Louis encephalitis virus;SBV : Schmallenberg virus;SHUV : Shuni virus;THOV : Thogoto virus;VEE : Venezuelan equine encephalitis virus;VSV : Vesicular stomatitis virus;WSLV : Wesselsbron virus;WNV : West Nile fever virus;WEEV : Western equine encephalitis virus;YUOV : Yunnan orbivirus virus.
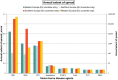
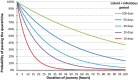
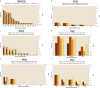
Risk scores: very low annual extent of spread: 0–0.2; low annual extent of spread: 0.2–0.4; moderate annual extent of spread: 0.4–0.6, high annual extent of spread: 0.6–0.8, very high annual extent of spread: > 0.8. The annual extent of spread is a logarithmic translation of the number of infected hosts that is expected to develop within one year. A risk score of 0 translates to 1 host, a score of 0.2 translates to 10 hosts, a score of 0.4 translates to 100 hosts, etc. and a score of 1 translates to 100,000 hosts. The annual extent of spread score (sc) translates into the number of infected hosts (units)/year (# infected hosts/year) using the following formula: # infected hosts/year = 10^[5 × sc].
BTV : Bluetongue virus;CVV : Bunyamwera virus;CCHFV : Crimean Congo haemorrhagic fever virus;EEEV : Eastern equine encephalitis virus; Hepatozoonis; (H. canis); CanL: Leishmaniosis (L. infantum);SBV : Schmallenberg virus;WNV : West Nile virus.
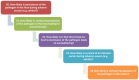
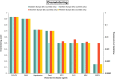
Risk scores: very low probability of overwintering: 0‐0.2; low probability of overwintering: 0.2–0.4; moderate probability of overwintering: 0.4–0.6, high probability of overwintering: 0.6–0.8, very high probability of overwintering: > 0.8. The overwintering score translates into a probability that the infection will persist through the winter. An overwintering score of 0.8 stands for nearly certain persistence through the winter, if the epidemic enters the winter with about 100 infected hosts/vectors, a score of 0.6 stands for a probability of 10%, a score of 0.4 stands for a probability of 1%. The overwintering score (sc) translates into the overwintering probability using the following formula: overwintering probability = 10^[5 × (sc − 1)].
BTV : Bluetongue virus;CVV : Bunyamwera virus;CCHFV : Crimean Congo haemorrhagic fever virus;EEEV : Eastern equine encephalitis virus; Hepatozoonis; (H. canis); CanL: Leishmaniosis (L. infantum);SBV : Schmallenberg virus;WNV : West Nile virus.

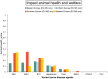
Impact scores: very low impact: 0–0.2; low impact: 0.2–0.4; moderate impact: 0.4–0.6, high impact: 0.6–0.8, very high impact: > 0.8.
BTV : Bluetongue virus;CVV : Bunyamwera virus;CCHFV : Crimean Congo haemorrhagic fever virus;EEEV : Eastern equine encephalitis virus; Hepatozoonis; (H. canis); CanL: Leishmaniosis (L. infantum);SBV : Schmallenberg virus;WNV : West Nile virus.
References
-
- Amela C, Mendez I, Torcal JM, Medina G, Pachon I, Canavate C and Alvar J, 1995. Epidemiology of canine leishmaniasis in the Madrid region, Spain. European Journal of Epidemiology, 11, 157–161. - PubMed
LinkOut - more resources
Full Text Sources
