Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018)
- PMID: 32625771
- PMCID: PMC7009685
- DOI: 10.2903/j.efsa.2018.5494
Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018)
Abstract
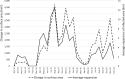
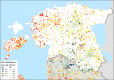
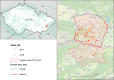
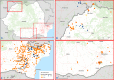
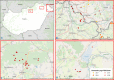
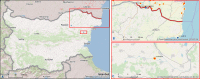
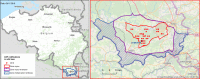
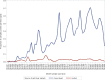
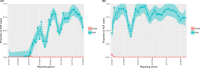
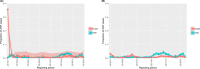
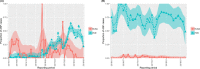
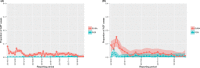
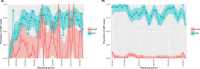
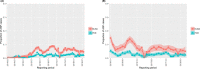
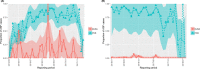
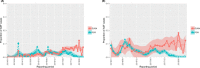
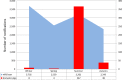
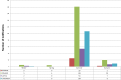
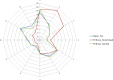
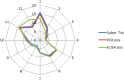
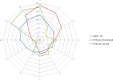
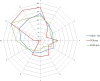
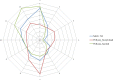
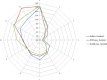
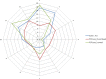
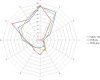
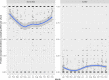
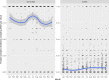
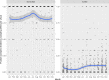
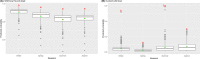
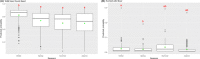
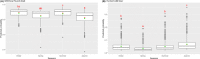
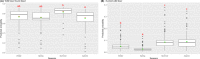
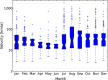
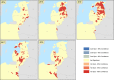
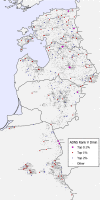
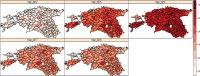
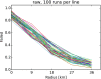
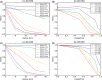
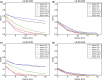
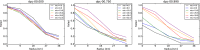
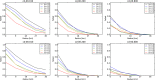
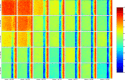
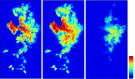
This update on the African swine fever (ASF) outbreaks in the EU demonstrated that out of all tested wild boar found dead, the proportion of positive samples peaked in winter and summer. For domestic pigs only, a summer peak was evident. Despite the existence of several plausible factors that could result in the observed seasonality, there is no evidence to prove causality. Wild boar density was the most influential risk factor for the occurrence of ASF in wild boar. In the vast majority of introductions in domestic pig holdings, direct contact with infected domestic pigs or wild boar was excluded as the route of introduction. The implementation of emergency measures in the wild boar management zones following a focal ASF introduction was evaluated. As a sole control strategy, intensive hunting around the buffer area might not always be sufficient to eradicate ASF. However, the probability of eradication success is increased after adding quick and safe carcass removal. A wider buffer area leads to a higher success probability; however it implies a larger intensive hunting area and the need for more animals to be hunted. If carcass removal and intensive hunting are effectively implemented, fencing is more useful for delineating zones, rather than adding substantially to control efficacy. However, segments of fencing will be particularly useful in those areas where carcass removal or intensive hunting is difficult to implement. It was not possible to demonstrate an effect of natural barriers on ASF spread. Human-mediated translocation may override any effect of natural barriers. Recommendations for ASF control in four different epidemiological scenarios are presented.
Keywords: African swine fever; domestic pigs; epidemiology; management; prevention; risk factor; seasonality; wild boar.
© 2018 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures

















References
-
- Albrycht M, Merta D, Bobek J and Ulejczyk S, 2016. The demographic pattern of wild boars (Sus scrofa) inhabiting fragmented forest in north‐eastern Poland. Baltic Forestry, 22, 251–258.
-
- BFSA (Bulgarian Food Safety Agency), 2018. African swine fever control measures in Bulgaria. Presentation at the PAFF meeting, 12–13 July 2018, Brussels. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_2018... [Accessed: 31 October 2018].
LinkOut - more resources
Full Text Sources
Research Materials
