Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009
- PMID: 32625944
- PMCID: PMC7009395
- DOI: 10.2903/j.efsa.2018.5311
Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009
Abstract
This Guidance describes how to perform hazard identification for endocrine-disrupting properties by following the scientific criteria which are outlined in Commission Delegated Regulation (EU) 2017/2100 and Commission Regulation (EU) 2018/605 for biocidal products and plant protection products, respectively.
Keywords: biocidal product; endocrine disruptor; guidance; hazard identification; plant protection product.
© 2018 European Chemicals Agency and © European Food Safety Authority.
Figures
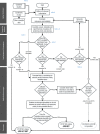
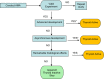
Notes to Figure 1: The assessment strategy illustrated in the flow chart is applicable both for humans and non‐target organisms and in both cases the guidance specified in Sections 3.2–3.5 of this document needs to be followed. The assessment strategy is driven by the availability of ‘
EATS ‐mediated’ parameters as these provide evidence for both endocrine activity and the resulting potentially adverse effects. However, there may be situations where the ‘EATS ‐mediated’ parameters are not, sufficiently, investigated (e.g. this is very likely the case for non‐target organisms). In such cases, it may be possible to follow the assessment strategy using the ‘sensitive to, but not diagnostic of,EATS ’ parameters (without the need to generate additional information onEATS ‐mediated parameters i.e. in case of scenarios 2a(i) or 2b). If the required data are available, it is in principle possible to establish endocrine disrupting MoA(s) on the basis of parameters indicating ‘sensitive to, but not diagnostic of,EATS ’ potential adversity andEATS endocrine activity.Note a : According to theED criteria forBCP s ‘scientific data generated in accordance with internationally agreed study protocols, in particular those referred to in AnnexesII andIII of Regulation (EU ) No 528/2012’; according to theED criteria forPPP ‘scientific data generated in accordance with internationally agreed study protocols, in particular, those listed in the Commission Communications in the framework of setting out the data requirements for active substances and plant protection products, in accordance with this Regulation’.Note b : As discussed in Section 3.1, some substances may not need to be assessed forED properties.Note c : For details on population relevance see Section 3.3.1.4.Note d : For details on effects secondary to other toxicities see Section 3.3.1.1.Note e : If information on non‐EATS modalities becomes available, e.g. through systematic review of the literature, this needs be taken forward in the assessment. In such cases, after gathering and assessing the information, and assembling and reporting the lines of evidence (see Section 3.3), the non‐EATS information can be taken forward directly to the MoA analysis (the step ‘initial analysis of the evidence’ is not applicable), as explained in Section 3.5.Note f : For details onEATS ‐mediated adversity considered sufficiently investigated see Section 3.4.Note g : For details on endocrine activity considered sufficiently investigated: see Section 3.4.2 and 3.4.3.

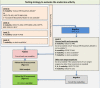
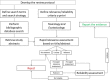
Note a: The ToxCast Estrogen Receptor (
ER ) model integrates multiple in vitro assays, high‐throughput ToxCast screenings assays measuring receptor (ER ) binding, dimerisation, chromatin binding, transcriptional activation andER ‐dependent cell proliferation (Judson et al., 2015). The multiple in vitro assays provide comprehensive pathway coverage for the biology of theER signalling pathway (Browne et al., 2015).US EPA is accepting ToxCastER model for 1812 chemicals as alternatives forEDSP tier 1ER binding,ER transactivation, and uterotrophic assays.Note b: Partially covered by the
OECD TG 441. However, it is recommended to always investigate the S modality with the E and A modalities and not to interpret negative results in isolation.Note c: Level 2 tests and, for mammals, specific level 3 tests are not yet available. The T modality is included in this figure for completeness. To consider the T modality as ‘sufficiently investigated’ for human health and mammals the thyroid parameters from
OECD test guidelines 407, 408, 409 (and/or the one‐year dog study, if available), 416 (or 443 if available) and 451‐3 should have been measured (see Section 3.4.1).
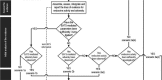

KE : key event;MIE : molecular initiating event.
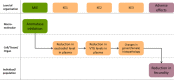
*Histology may be required by some regulatory authorities despite significant differences in advanced and asynchronous development. The entity performing this test is encouraged to consult the competent authorities prior to performing the test to determine which parameters are required.
References
-
- Adori M, Kiss E, Barad Z, Barabas K, Kiszely E, Schneider A, Kovesdi D, Sziksz E, Abraham IM, Matko J and Sarmay G, 2010. Estrogen augments the T cell‐dependent but not the T‐independent immune response. Cellular and Molecular Life Sciences 67, 1661–1674. 10.1007/s00018-010-0270-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
