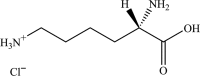
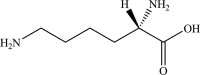
Safety and efficacy of l-lysine monohydrochloride and concentrated liquid l-lysine (base) produced by fermentation using Corynebacterium glutamicum strain NRRL B-50775 for all animal species based on a dossier submitted by ADM
- PMID: 32626082
- PMCID: PMC7009140
- DOI: 10.2903/j.efsa.2019.5537
Safety and efficacy of l-lysine monohydrochloride and concentrated liquid l-lysine (base) produced by fermentation using Corynebacterium glutamicum strain NRRL B-50775 for all animal species based on a dossier submitted by ADM
Abstract
The European Commission asked EFSA for an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of a l-lysine monohydrochloride (HCl, minimum 98.5%) and of a concentrated liquid l-lysine (base, minimum 50%) produced by a genetically modified strain of Corynebacterium glutamicum (NRRL B-50775). They are intended to be used in feed or water for drinking for all animal species and categories. Neither the production strain C. glutamicum NRRL B-50775 nor its recombinant DNA was detected in the final product. Therefore, the product does not pose any safety concern associated with the genetic modification of the production strain. l-Lysine HCl and concentrated liquid l-lysine (base) produced by C. glutamicum NRRL B-50775 are considered safe for the target species, for the consumer and for the environment. l-Lysine HCl produced by C. glutamicum NRRL B-50775 is considered not irritant to skin or eyes and not a skin sensitiser. In the absence of data, the FEEDAP Panel cannot conclude on the potential toxicity by inhalation of l-lysine HCl produced by C. glutamicum NRRL B-50775. Concentrated liquid l-lysine (base) produced by C. glutamicum NRRL B-50775, due to its high pH (11) it is anticipated to be corrosive to skin and eyes and poses a risk by inhalation. l-Lysine HCl and concentrated liquid l-lysine (base) produced by C. glutamicum NRRL B-50775 are considered as efficacious sources of the essential amino acid l-lysine for non-ruminant animal species. For the supplemental l-lysine to be as efficacious in ruminants as in non-ruminant species, it would require protection against degradation in the rumen.
Keywords: amino acids; concentrated liquid l‐lysine base; l‐lysine monohydrochloride; nutritional additive; safety.
© 2019 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
References
-
- Broderick GA and Balthrop JE, 1979. Chemical inhibition of amino acid deamination by ruminal microbes in vitro. Journal of Animal Science, 49, 1101–1111.
-
- Chalupa W, 1976. Degradation of amino acids by the mixed rumen microbial population. Journal of Animal Science, 43, 828–834. - PubMed
-
- EFSA (European Food Safety Authority), 2007. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the safety and efficacy of L‐lysine sulphate (Vitalys®Liquid and Vitalys®Dry) for all animal species. The EFSA Journal 2007;5(9):522, 26 pp. 10.2903/j.efsa.2007.522 - DOI
-
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(8):587, 16 pp. 10.2903/j.efsa.2007.587 - DOI
-
- EFSA (European Food Safety Authority), 2008a. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 - DOI
LinkOut - more resources
Full Text Sources