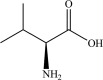
Safety and efficacy of l-valine produced by fermentation using Corynebacterium glutamicum KCCM 11201P for all animal species
- PMID: 32626083
- PMCID: PMC7009307
- DOI: 10.2903/j.efsa.2019.5538
Safety and efficacy of l-valine produced by fermentation using Corynebacterium glutamicum KCCM 11201P for all animal species
Abstract
The product subject of this assessment is l-valine produced by fermentation using a non-genetically modified strain of Corynebacterium glutamicum (KCCM 11201P). It is intended to be used in feed and water for drinking for all animal species and categories. Species identity of the production organism was confirmed and the strain was sensitive to antibiotics at concentrations below thresholds specified by EFSA, thus C. glutamicum KCCM 11201P may be considered safe by the qualified presumption of safety (QPS) approach. No viable cells of C. glutamicum were detected in the final product. The amount of identified material exceeded ■■■■■% on 'as is' basis, and no impurities of concern were detected. The use of l-valine produced by C. glutamicum KCCM 11201P is safe for target species when supplemented to diets in appropriate amounts, for the consumer and the environment. The product l-valine produced by C. glutamicum (KCCM 11201P) is considered not to be an irritant or a dermal sensitiser, and does not cause acute inhalation toxicity. The additive is an effective source of valine for all species. For the supplemental l-valine to be as efficacious in ruminants as in non-ruminant species, it requires protection against microbial degradation in the rumen.
Keywords: C. glutamicum KCCM 11201P; amino acids; efficacy; l‐valine; nutritional additive; safety.
© 2019 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
References
-
- Broderick GA and Balthrop JE, 1979. Chemical inhibition of amino acid deamination by ruminal microbes in vitro . Journal of Animal Science, 49, 1101‐1111.
-
- CIR (Cosmetic Ingredient Review), 2012. Safety Assessment of α‐Amino Acids as Used in Cosmetics. Available online: http://www.cir-safety.org/sites/default/files/amacid092012rep.pdf
-
- Chalupa W, 1976. Degradation of amino acids by the mixed rumen microbial population. Journal of Animal Science, 43, 828–834. - PubMed
-
- EFSA (European Food Safety Authority), 2008a. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on the safety of amino acids from Chemical Group 34, Flavouring Group Evaluation 26, Revision 1. EFSA Journal 2008;6(8):790, 51 pp. 10.2903/j.efsa.2008.790 - DOI
-
- EFSA (European Food Safety Authority), 2008b. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 - DOI
LinkOut - more resources
Full Text Sources