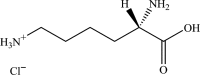
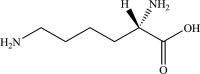
Safety and efficacy of l-lysine monohydrochloride and concentrated liquid l-lysine (base) produced by fermentation using Corynebacterium glutamicum strains NRRL-B-67439 or NRRL B-67535 for all animal species
- PMID: 32626174
- PMCID: PMC7008832
- DOI: 10.2903/j.efsa.2019.5886
Safety and efficacy of l-lysine monohydrochloride and concentrated liquid l-lysine (base) produced by fermentation using Corynebacterium glutamicum strains NRRL-B-67439 or NRRL B-67535 for all animal species
Abstract
The European Commission asked EFSA for an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of a l-lysine monohydrochloride (HCl, minimum 98.5%) and of a concentrated liquid l-lysine (base, minimum 50%) produced by genetically modified strains of Corynebacterium glutamicum (NRRL-B-67439 or NRRL B-67535). They are intended to be used in feed or water for drinking for all animal species and categories. Neither viable cells of the production strains C. glutamicum strains NRRLB-67439 or NRRL B-67535; nor their recombinant DNA were detected in the final products. Therefore, those products do not pose any safety concern associated with the genetic modification of the production strains. l-Lysine HCl and concentrated liquid l-lysine (base) produced by C. glutamicum strains NRRLB-67439 or NRRL B-67535 are considered safe for the target species, for the consumer and for the environment. l-Lysine HCl produced by C. glutamicum strains NRRL B-67439 or NRRL B-67535 is considered not irritant to skin or eyes and not a skin sensitiser. In the absence of data, the FEEDAP Panel cannot conclude on the potential toxicity by inhalation of l-lysine HCl produced by C. glutamicum strains NRRL B-67439 or NRRL B-67535. Concentrated liquid l-lysine (base) produced by C. glutamicum strains NRRL B-67439 or NRRL B-67535, due to its high pH (10.7 and 10.9, respectively) is anticipated to be corrosive to skin and eyes and poses a risk by inhalation. l-Lysine HCl and concentrated liquid l-lysine (base) produced by C. glutamicum strains NRRLB-67439 or NRRL B-67535 are considered as efficacious sources of the essential amino acid l-lysine for non-ruminant animal species. For the supplemental l-lysine to be as efficacious in ruminants as in non-ruminant species, it would require protection against degradation in the rumen.
Keywords: Corynebacterium glutamicum; amino acid; efficacy; lysine base; lysine monohydrochloride; nutritional additive; safety.
© 2019 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
References
-
- EFSA (European Food Safety Authority), 2007. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the safety and efficacy of l‐lysine sulphate (Vitalys®Liquid and Vitalys®Dry) for all animal species. EFSA Journal 2007;5(9):522, 26 pp. 10.2903/j.efsa.2007.522 - DOI
-
- EFSA (European Food Safety Authority), 2008. Scientific opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC). Amino acids from chemical group 34 Flavouring Group Evaluation 26, Revision 1. EFSA Journal 2008;6(8):790, 51 pp. 10.2903/j.efsa.2008.790 - DOI - PMC - PubMed
-
- EFSA AFC Panel (Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food), 2010. Scientific opinion on Flavouring Group Evaluation 26: Amino acids from chemical group 34. EFSA Journal 2010;8(12):373, 48 pp. 10.2903/j.efsa.2006.373 - DOI
-
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernandez Escamez PS, Maradona MP, Querol A, Suarez JE, Sundh I, Vlak J, Barizzone F, Correia S and Herman L, 2019. Statement on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 9: suitability of taxonomic units notified to EFSA until September 2019. EFSA Journal 2019;17(1):5555, 46 pp. 10.2903/j.efsa.2019.5555 - DOI - PMC - PubMed
-
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011. Scientific Opinion on Flavouring Group Evaluation 96 (FGE.96): Consideration of 88 flavouring substances considered by EFSA for which EU production volumes/anticipated production volumes have been submitted on request by DG SANCO. Addendum to FGE. 51, 52, 53, 54, 56, 58, 61, 62, 63, 64, 68, 69, 70, 71, 73, 76, 77, 79, 80, 83, 84, 85 and 87. EFSA Journal 2011;9(12):1924, 60 pp. 10.2903/j.efsa.2011.1924 - DOI
LinkOut - more resources
Full Text Sources