Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals
- PMID: 32626259
- PMCID: PMC7009070
- DOI: 10.2903/j.efsa.2019.5634
Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals
Abstract
This Guidance document describes harmonised risk assessment methodologies for combined exposure to multiple chemicals for all relevant areas within EFSA's remit, i.e. human health, animal health and ecological areas. First, a short review of the key terms, scientific basis for combined exposure risk assessment and approaches to assessing (eco)toxicology is given, including existing frameworks for these risk assessments. This background was evaluated, resulting in a harmonised framework for risk assessment of combined exposure to multiple chemicals. The framework is based on the risk assessment steps (problem formulation, exposure assessment, hazard identification and characterisation, and risk characterisation including uncertainty analysis), with tiered and stepwise approaches for both whole mixture approaches and component-based approaches. Specific considerations are given to component-based approaches including the grouping of chemicals into common assessment groups, the use of dose addition as a default assumption, approaches to integrate evidence of interactions and the refinement of assessment groups. Case studies are annexed in this guidance document to explore the feasibility and spectrum of applications of the proposed methods and approaches for human and animal health and ecological risk assessment. The Scientific Committee considers that this Guidance is fit for purpose for risk assessments of combined exposure to multiple chemicals and should be applied in all relevant areas of EFSA's work. Future work and research are recommended.
Keywords: combined exposure to multiple chemicals; dose addition; harmonised methodologies; interactions; mixtures; response addition; risk assessment.
© 2019 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures


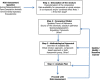
Central in red are specific aspects required for risk assessment of combined exposure to multiple chemicals; these factors require attention and/or decisions for all assessment steps in an iterative way. WMA: whole mixture approach; CBA: component‐based approach; UF: Uncertainty factors; RC: risk characterisation; DA: dose addition.

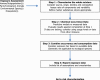
Note: Occurrence and consumption data ranges from default values (tier 0) to individual co‐occurrence data and individual data, respectively (tier 3), and consequently, exposure estimates range from semi‐quantitative point estimates (tier 0) to probabilistic (tier 3). Occurrence and consumption tiers do not necessarily match.


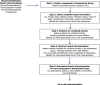
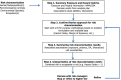

References
-
- Altenburger R, Schmitt H and Schuurmann G, 2005. Algal toxicity of nitrobenzenes: Combined effect analysis as a pharmacological probe for similar modes of interaction. Environmental Toxicology and Chemistry, 24, 324–333. - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE and Villeneuve DL, 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry, 29, 730–741. - PubMed
-
- ATSDR (Agency for Toxic Substances and Disease Registry), 2004. Guidance Manual for the Assessment of Joint Toxic Action of Chemical Mixtures. US Department of Health and Human Services, 107.
-
- ATSDR (Agency for Toxic Substances and Disease Registry), 2018. Framework for assessing health impacts of multiple chemicals and other stressors (update). Available online: https://www.atsdr.cdc.gov/interactionprofiles/ip-ga/ipga.pdf
-
- Azimonti G, Galimberti F, Marchetto F, Menaballi L, Ullucci S, Pellicioli F, Alessandra C, Lidia C, Alessio I, Angelo M, de Boer W and van der Voet H, 2015. Comparison of NOEC values to EC10/EC20 values, including confidence intervals, in aquatic and terrestrial ecotoxicological risk assessment. EFSA Supporting Publications, 12(12), p.906E.
LinkOut - more resources
Full Text Sources
