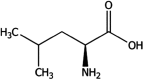
Safety and efficacy of l-leucine produced by fermentation with Escherichia coli NITE BP-02351 for all animal species
- PMID: 32626314
- PMCID: PMC7009086
- DOI: 10.2903/j.efsa.2019.5689
Safety and efficacy of l-leucine produced by fermentation with Escherichia coli NITE BP-02351 for all animal species
Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on l-leucine produced by fermentation with Escherichia coli NITE BP-02351 when used as nutritional additive or as feed flavouring compound in feed and water for drinking for all animal species. The product under assessment is l-leucine produced by fermentation with a genetically modified strain of E. coli (NITE BP-02351). The production strain and its recombinant DNA were not detected in the final products. l-Leucine, manufactured by fermentation with E. coli NITE BP-02351, does not give rise to any safety concern to the production strain. The use of l-leucine produced with E. coli NITE BP-02351 is safe for the target species when used to supplement the diet in appropriate amounts. It is safe at the proposed use level of 25 mg/kg when used as flavouring compound for all animal species. The use of l-leucine produced by fermentation with E. coli NITE BP-02351 in animal nutrition raises no safety concerns for consumers of animal products. The additive is not irritating to the skin or eyes and is not a skin sensitiser. There is a risk for persons handling the additive from the exposure to endotoxins by inhalation. The use of l-leucine produced by E. coli NITE BP-02351 as feed additive does not represent a risk to the environment. The additive l-leucine produced by E. coli NITE BP-02351 is regarded as an effective source of the amino acid l-leucine when used as nutritional additive. For the supplemental l-leucine to be as efficacious in ruminants as in non-ruminant species, it requires protection against degradation in the rumen. It is also considered efficacious as feed flavouring compound under the proposed conditions of use.
Keywords: Escherichia coli NITE BP 02351; amino acid; flavouring; leucine; nutritional additive; sensory additive.
© 2019 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
References
-
- Baker DH, 2005. Tolerance for branched‐chain amino acids in experimental animals and humans. Journal of Nutrition, 135, 1585–1590. - PubMed
-
- ■■■■■
-
- Blackburn TH, 1965. Nitrogen metabolism in the rumen. In: Physiology of Digestion in the Ruminant. Ed. By Dougherty et al., Butterworths, Washington, 1965. 322–334.
-
- Broderick GA and Balthrop JE, 1979. Chemical inhibition of amino acid deamination by ruminal microbes in vitro. Journal of Animal Science, 49, 1101–1111.
-
- ■■■■■