Potent Tetrahydroquinolone Eliminates Apicomplexan Parasites
- PMID: 32626661
- PMCID: PMC7311950
- DOI: 10.3389/fcimb.2020.00203
Potent Tetrahydroquinolone Eliminates Apicomplexan Parasites
Abstract
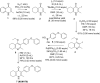
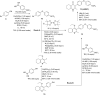
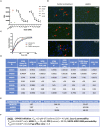
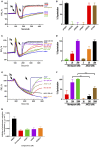
Apicomplexan infections cause substantial morbidity and mortality, worldwide. New, improved therapies are needed. Herein, we create a next generation anti-apicomplexan lead compound, JAG21, a tetrahydroquinolone, with increased sp3-character to improve parasite selectivity. Relative to other cytochrome b inhibitors, JAG21 has improved solubility and ADMET properties, without need for pro-drug. JAG21 significantly reduces Toxoplasma gondii tachyzoites and encysted bradyzoites in vitro, and in primary and established chronic murine infections. Moreover, JAG21 treatment leads to 100% survival. Further, JAG21 is efficacious against drug-resistant Plasmodium falciparum in vitro. Causal prophylaxis and radical cure are achieved after P. berghei sporozoite infection with oral administration of a single dose (2.5 mg/kg) or 3 days treatment at reduced dose (0.625 mg/kg/day), eliminating parasitemia, and leading to 100% survival. Enzymatic, binding, and co-crystallography/pharmacophore studies demonstrate selectivity for apicomplexan relative to mammalian enzymes. JAG21 has significant promise as a pre-clinical candidate for prevention, treatment, and cure of toxoplasmosis and malaria.
Keywords: Plasmodium falciparum; RPS13Δ; Toxoplasma gondii; cytochrome bc1; nanoformulation; structure-guided design; tetrahydroquinolone; transcriptomics.
Copyright © 2020 McPhillie, Zhou, Hickman, Gordon, Weber, Li, Lee, Amporndanai, Johnson, Darby, Woods, Li, Priestley, Ristroph, Biering, El Bissati, Hwang, Hakim, Dovgin, Lykins, Roberts, Hargrave, Cong, Sinai, Muench, Dubey, Prud'homme, Lorenzi, Biagini, Moreno, Roberts, Antonyuk, Fishwick and McLeod.
Figures




References
-
- Bradbury R. H., Allot C. P., Dennis M., Girdwood J. A., Kenny P. W., Major J. S., et al. . (1993). New nonpeptide angiotensin II receptor antagonists. 3. Synthesis, biological properties, and structure-activity relationships of 2-alkyl-4-(biphenylylmethoxy)pyridine derivatives. J. Med. Chem. 36, 1245–1254. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 AI147661/AI/NIAID NIH HHS/United States
- MC_PC_17167/MRC_/Medical Research Council/United Kingdom
- MR/L000644/1/MRC_/Medical Research Council/United Kingdom
- R01 AI145335/AI/NIAID NIH HHS/United States
- R01 AI027530/AI/NIAID NIH HHS/United States
- R01 AI128356/AI/NIAID NIH HHS/United States
- U19 AI110819/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 AI082180/AI/NIAID NIH HHS/United States
- R01 AI071319/AI/NIAID NIH HHS/United States
- U01 AI077887/AI/NIAID NIH HHS/United States
- HHSN272200900007C/AI/NIAID NIH HHS/United States
- 109158/B/15/Z/WT_/Wellcome Trust/United Kingdom
- T35 DK062719/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

