Nasopharyngeal Swabs Are More Sensitive Than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load
- PMID: 32626720
- PMCID: PMC7314917
- DOI: 10.3389/fmed.2020.00334
Nasopharyngeal Swabs Are More Sensitive Than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load
Abstract
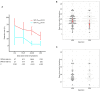
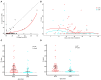
Objective: Detection of SARS-CoV-2 by oropharyngeal swabs (OPS) and nasopharyngeal swabs (NPS) is an essential method for coronavirus disease 2019 (COVID-19) management. It is not clear how detection rate, sensitivity, and the risk of exposure for medical providers differ in two sampling methods. Methods: In this prospective study, 120 paired NPS and OPS specimens were collected from 120 inpatients with confirmed COVID-19. SARS-CoV-2 nucleic acid in swabs were detected by real-time RT-PCR. The SARS-CoV-2 detection rate, sensitivity, and viral load were analyzed with regards NPS and OPS. Sampling discomfort reported by patients was evaluated. Results: The SARS-CoV-2 detection rate was significantly higher for NPS [46.7% (56/120)] than OPS [10.0% (12/120)] (P < 0.001). The sensitivity of NPS was also significantly higher than that of OPS (P < 0.001). At the time of sampling, the time of detectable SARS-CoV-2 had a longer median duration (25.0 vs. 20.5 days, respectively) and a longer maximum duration (41 vs. 39 days, respectively) in NPS than OPS. The mean cycle threshold (Ct) value of NPS (37.8, 95% CI: 37.0-38.6) was significantly lower than that of OPS (39.4, 95% CI: 38.9-39.8) by 1.6 (95% CI 1.0-2.2, P < 0.001), indicating that the SARS-CoV-2 load was significantly higher in NPS specimens than OPS. Patient discomfort was low in both sampling methods. During NPS sampling, patients were significantly less likely to have nausea and vomit. Conclusions: NPS had significantly higher SARS-CoV-2 detection rate, sensitivity, and viral load than OPS. NPS could reduce droplets production during swabs. NPS should be recommended for diagnosing COVID-19 and monitoring SARS-CoV-2 load. Chinese Clinical Trial Registry, number: ChiCTR2000029883.
Keywords: COVID-19; SARS-CoV-2; nasopharyngeal swab; oropharyngeal swab; sensitivity; viral load.
Copyright © 2020 Wang, Liu, Hu, Zhou, Yu, Li, Xu, Xiao, Yang, Lu, Wang, Yin and Xu.
Figures

References
-
- World Health Organization Coronavirus Disease (COVID-2019) Situation Reports. (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio... (accessed April 20, 2020).
LinkOut - more resources
Full Text Sources
Miscellaneous

