Cyclic stretch promotes the ossification of ligamentum flavum by modulating the Indian hedgehog signaling pathway
- PMID: 32626952
- PMCID: PMC7339599
- DOI: 10.3892/mmr.2020.11200
Cyclic stretch promotes the ossification of ligamentum flavum by modulating the Indian hedgehog signaling pathway
Abstract
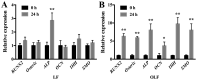
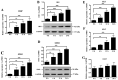
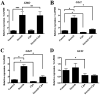
The Indian hedgehog (IHH) signaling pathway is an important pathway for bone growth and development. The aim of the present study was to examine the role of the IHH signaling pathway in the development of the ossification of ligamentum flavum (OLF) at the cellular and tissue levels. The expression levels and localization of the osteogenic genes Runt-related transcription factor 2 (RUNX2), Osterix, alkaline phosphatase (ALP), osteocalcin (OCN) and IHH were evaluated in OLF tissues by reverse transcription-quantitative PCR (RT-qPCR) and immunohistochemistry. Non-ossified ligamentum flavum (LF) sections were used as control samples. The tissue explant method was used to obtain cultured LF cells. In addition, OLF cells were subjected to cyclic stretch application for 0, 6, 12 or 24 h. The expression levels of osteogenic genes, and the IHH signaling pathway genes IHH, Smoothened (SMO), GLI family zinc finger 1 (GLI1), GLI2 and GLI3 were evaluated with RT-qPCR and western blotting. Osteogenic differentiation was further evaluated by assessing ALP activity and staining. Moreover, the effect of cyclopamine (Cpn), an IHH signaling inhibitor, on osteogenic differentiation was examined. The RT-qPCR and immunohistochemical results indicated that the mRNA and protein expression levels of RUNX2, Osterix, ALP, OCN and IHH were significantly higher in the OLF group compared with the LF group. Furthermore, application of cyclic stretch to OLF cells resulted in greater ALP activity, and significant increases in mRNA and protein expression levels of RUNX2, Osterix, ALP and OCN in a time-d00ependent manner. Cyclic stretch application also led to significant increases in IHH signaling pathway genes, including IHH, SMO, GLI1 and GLI2, while no significant effect was found on GLI3 expression level. In addition, it was found that Cpn significantly reversed the effect of cyclic stretch on the ALP activity, and the expression levels of RUNX2, Osterix, ALP, OCN, GLI1 and GLI2. Collectively, the present results suggested that the IHH signaling pathway may mediate the effect of cyclic stretch on the OLF cells.
Keywords: igamentum flavum; cyclic stretch; ossification; osteogenic differentiation; pathway; Indian hedgehog.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

