Siva‑1 regulates multidrug resistance of gastric cancer by targeting MDR1 and MRP1 via the NF‑κB pathway
- PMID: 32626967
- PMCID: PMC7339453
- DOI: 10.3892/mmr.2020.11211
Siva‑1 regulates multidrug resistance of gastric cancer by targeting MDR1 and MRP1 via the NF‑κB pathway
Abstract
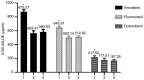
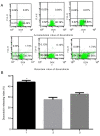
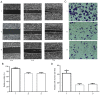
Siva‑1 is a well‑known anti‑apoptosis protein that serves a role in multiple types of cancer cells. However, whether Siva‑1 affects multidrug resistance via the NF‑κB pathway in gastric cancer is currently unknown. The present study aimed to determine the possible involvement of Siva‑1 in gastric cancer anticancer drug resistance in vitro. A vincristine (VCR)‑resistant KATO III/VCR gastric cancer cell line with stable Siva‑1 overexpression was established. The protein expression levels of Siva‑1, NF‑κB, multidrug resistance 1 (MDR1) and multidrug resistance protein 1 (MRP1) were detected via western blotting. The effect of Siva‑1 overexpression on anticancer drug resistance was assessed by measuring the 50% inhibitory concentration of KATO III/VCR cells to VCR, 5‑fluorouracil and doxorubicin. The rate of doxorubicin efflux and apoptosis were detected by flow cytometry. Additionally, colony formation, wound healing and Transwell assays were used to detect the proliferation, migration and invasion of cells, respectively. The results of the current study revealed that the Siva‑1‑overexpressed KATO III/VCR gastric cancer cells exhibited a significantly decreased sensitivity to VCR, 5‑fluorouracil and doxorubicin. The results of flow cytometry revealed that the percentage of apoptotic cells decreased following overexpression of Siva‑1. The colony formation assay demonstrated that cell growth and proliferation were significantly promoted by Siva‑1 overexpression. Additionally, Siva‑1 overexpression increased the migration and invasion of KATO III/VCR cells in vitro. Western blot analysis determined that Siva‑1 overexpression increased NF‑κB, MDR1 and MRP1 levels. The current study demonstrated that overexpression of Siva‑1, which functions as a regulator of MDR1 and MRP1 gene expression in gastric cancer cells via promotion of NF‑κB expression, inhibited the sensitivity of gastric cancer cells to certain chemotherapies. These data provided novel insight into the molecular mechanisms of gastric cancer, and may be of significance for the clinical diagnosis and therapy of patients with gastric cancer.
Keywords: Siva-1; multidrug resistance; gastric cancer.
Figures



References
-
- Zeng L, Liao Q, Zou Z, Wen Y, Wang J, Liu C, He Q, Weng N, Zeng J, Tang H, et al. Long non-coding RNA XLOC_006753 promotes the development of multidrug resistance in gastric cancer cells through the PI3K/AKT/mTOR signaling pathway. Cell Physiol Biochem. 2018;51:1221–1236. doi: 10.1159/000495499. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

