Clinical decision trees support systematic evaluation of multidisciplinary team recommendations
- PMID: 32627108
- PMCID: PMC7383031
- DOI: 10.1007/s10549-020-05769-1
Clinical decision trees support systematic evaluation of multidisciplinary team recommendations
Abstract
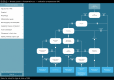
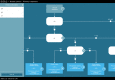
Purpose: EUSOMA's recommendation that "each patient has to be fully informed about each step in the diagnostic and therapeutic pathway" could be supported by guideline-based clinical decision trees (CDTs). The Dutch breast cancer guideline has been modeled into CDTs ( www.oncoguide.nl ). Prerequisites for adequate CDT usage are availability of necessary patient data at the time of decision-making and to consider all possible treatment alternatives provided in the CDT.
Methods: This retrospective single-center study evaluated 394 randomly selected female patients with non-metastatic breast cancer between 2012 and 2015. Four pivotal CDTs were selected. Two researchers analyzed patient records to determine to which degree patient data required per CDT were available at the time of multidisciplinary team (MDT) meeting and how often multiple alternatives were actually reported.
Results: The four selected CDTs were indication for magnetic resonance imaging (MRI) scan, preoperative and adjuvant systemic treatment, and immediate breast reconstruction. For 70%, 13%, 97% and 13% of patients, respectively, all necessary data were available. The two most frequent underreported data-items were "clinical M-stage" (87%) and "assessable mammography" (28%). Treatment alternatives were reported by MDTs in 32% of patients regarding primary treatment and in 28% regarding breast reconstruction.
Conclusion: Both the availability of data in patient records essential for guideline-based recommendations and the reporting of possible treatment alternatives of the investigated CDTs were low. To meet EUSOMA's requirements, information that is supposed to be implicitly known must be explicated by MDTs. Moreover, MDTs have to adhere to clear definitions of data-items in their reporting.
Keywords: Breast cancer; Clinical decision trees; Decision support; Guidelines; Multidisciplinary team.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Wilson AR, Marotti L, Bianchi S, Biganzoli L, Claassen S, Decker T, Frigerio A, Goldhirsch A, Gustafsson EG, Mansel RE, Orecchia R, Ponti A, Poortmans P, Regitnig P, Rosselli Del Turco M, Rutgers EJ, van Asperen C, Wells CA, Wengstrom Y, Cataliotti L, Eusoma The requirements of a specialist Breast Centre. Eur J Cancer. 2013;49(17):3579–3587. doi: 10.1016/j.ejca.2013.07.017. - DOI - PubMed
-
- Dutch National Breast Cancer Guideline (2012). https://www.oncoline.nl/richtlijn/item/index.php?pagina=/richtlijn/item/.... Accessed 13 Feb 2012
-
- Hendriks MP, Verbeek X, van Vegchel T, van der Sangen MJC, Strobbe LJA, Merkus JWS, Zonderland HM, Smorenburg CH, Jager A, Siesling SS. Transformation of the national breast cancer guideline into data-driven clinical decision trees. JCO Clin Cancer Inform. 2019;3:1–14. doi: 10.1200/CCI.18.00150. - DOI - PMC - PubMed
-
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Ostlund P, Tranaeus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schunemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13. doi: 10.1016/j.jclinepi.2017.05.006. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

