Stereoselective Synthesis of Tropanes via a 6π-Electrocyclic Ring-Opening/ Huisgen [3+2]-Cycloaddition Cascade of Monocyclopropanated Heterocycles
- PMID: 32627302
- PMCID: PMC7589232
- DOI: 10.1002/anie.202006030
Stereoselective Synthesis of Tropanes via a 6π-Electrocyclic Ring-Opening/ Huisgen [3+2]-Cycloaddition Cascade of Monocyclopropanated Heterocycles
Abstract
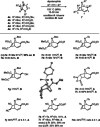
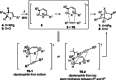
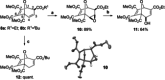
The synthesis of tropanes via a microwave-assisted, stereoselective 6π-electrocyclic ring-opening/ Huisgen [3+2]-cycloaddition cascade of cyclopropanated pyrrole and furan derivatives with electron-deficient dipolarophiles is demonstrated. Starting from furans or pyrroles, 8-aza- and 8-oxabicyclo[3.2.1]octanes are accessible in two steps in dia- and enantioselective pure form, being versatile building blocks for the synthesis of pharmaceutically relevant targets, especially for new cocaine analogues bearing various substituents at the C-6/C-7 positions of the tropane ring system. Moreover, the 2-azabicyclo[2.2.2]octane core (isoquinuclidines), being prominently represented in many natural and pharmaceutical products, is accessible via this approach.
Keywords: bicyclo[3.2.1]octanes; cocaine analogues; furans and pyrroles; isoquinuclidines; microwave-assisted [3+2]-cycloaddition; tropanes.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources

