Tocilizumab in patients with severe COVID-19: A single-center observational analysis
- PMID: 32628003
- PMCID: PMC7323415
- DOI: 10.1002/jmv.26191
Tocilizumab in patients with severe COVID-19: A single-center observational analysis
Abstract
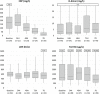
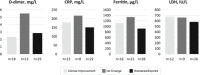
Patients with coronavirus disease 2019 (COVID-19) may develop severe respiratory distress, thought to be mediated by cytokine release. Elevated proinflammatory markers have been associated with disease severity. Tocilizumab, an interleukin-6 receptor antagonist, may be beneficial for severe COVID-19, when cytokine storm is suspected. This is a retrospective single-center analysis of the records of patients diagnosed with COVID-19 who received tocilizumab. Outcomes, including clinical improvement, mortality and changes in oxygen-support at 24, 48, and 72 hours, and 7, 14, and 28 days post-tocilizumab, are reported. Patients were evaluated by baseline pre-tocilizumab oxygenation status and changes in proinflammatory markers within 7 days post-tocilizumab are reported. Sixty-six patients received tocilizumab at a mean dose of 724 mg (7.4 mg/kg), 3.7 days from admission. At baseline, 53% of patients were on ventilation support and all had elevated proinflammatory markers, including c-reactive protein (CRP). Common comorbidities were diabetes mellitus (43%) and hypertension (74%). Most patients received concomitant glucocorticoids and hydroxychloroquine. Seven days after tocilizumab, ten patients (15.2%) had clinical improvement in their oxygenation status, and there was a 95% decrease in CRP. Within 14 days of treatment, 29% of patients had clinical improvement, 20% had minimal or no improvement, 17% worsened, 27% died, and 7% were transferred to an outside hospital. Ultimately, 42% of all patients that received tocilizumab expired and 49% were discharged. This study found limited clinical improvement in patients that received tocilizumab in the setting of severe COVID-19. Clinical trials are ongoing to further evaluate tocilizumab's benefit in this patient population.
Keywords: COVID-19; IL-6 antagonist; cytokine release syndrome; cytokine storm; tocilizumab.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
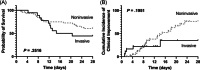
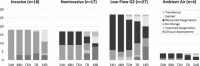
References
-
- World Health Organization . Coronavirus Disease 2019 (COVID‐19) Situation Report 51. March 11, 2020. Geneva, Switzerland: Who Health Organization; 2020.
-
- Cases in the US . 8 May 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed May 8 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

