Adherence to guideline-directed medical and device Therapy in outpAtients with heart failure with reduced ejection fraction: The ATA study
- PMID: 32628147
- PMCID: PMC7414820
- DOI: 10.14744/AnatolJCardiol.2020.91771
Adherence to guideline-directed medical and device Therapy in outpAtients with heart failure with reduced ejection fraction: The ATA study
Abstract
Objective: Despite recommendations from heart failure guidelines on the use of pharmacologic and device therapy in patients with heart failure with reduced ejection fraction (HFrEF), important inconsistencies in guideline adherence persist in practice. The aim of this study was to assess adherence to guideline-directed medical and device therapy for the treatment of patients with chronic HFrEF (left ventricular ejection fraction ≤40%).
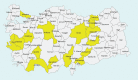
Methods: The Adherence to guideline-directed medical and device Therapy in outpAtients with HFrEF (ATA) study is a prospective, multicenter, observational study conducted in 24 centers from January 2019 to June 2019.
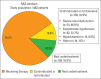
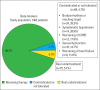
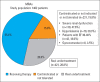
Results: The study included 1462 outpatients (male: 70.1%, mean age: 67±11 years, mean LVEF: 30%±6%) with chronic HFrEF. Renin-angiotensin system (RAS) inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRAs), and ivabradin were used in 78.2%, 90.2%, 55.4%, and 12.1% of patients, respectively. The proportion of patients receiving target doses of medical treatments was 24.6% for RAS inhibitors, 9.9% for beta-blockers, and 10.5% for MRAs. Among patients who met the criteria for implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy (CRT), only 16.9% of patients received an ICD (167 of 983) and 34% (95 of 279) of patients underwent CRT (95 of 279).
Conclusion: The ATA study shows that most HFrEF outpatients receive RAS inhibitors and beta-blockers but not MRAs or ivabradin when the medical reasons for nonuse, such as drug intolerance or contraindications, are taken into account. In addition, most eligible patients with HFrEF do not receive target doses of pharmacological treatments or guideline-recommended device therapy.
Conflict of interest statement
Figures
References
-
- Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, et al. Heart Failure Association of ESC (HFA) EURObservational Research Programme:the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2010;12:1076–84. - PubMed
-
- United Nations. Department of Economic and Social Affairs, Population Division. World population prospects:the 2008 revision. United Nations. 2009
-
- Değertekin M, Erol C, Ergene O, Tokgözoğlu L, Aksoy M, Erol MK, et al. Heart failure prevalence and predictors in Turkey:HAPPY study. Turk Kardiyol Dern Ars. 2012;40:298–308. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure:The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure:executive summary:a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

