Clinical Validity of Serum Antibodies to SARS-CoV-2 : A Case-Control Study
- PMID: 32628534
- PMCID: PMC7370852
- DOI: 10.7326/M20-2889
Clinical Validity of Serum Antibodies to SARS-CoV-2 : A Case-Control Study
Abstract
Background: The clinical utility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies remains undefined.
Objective: To determine the clinical validity and utility of SARS-CoV-2 antibodies.
Design: Case-control study.
Setting: First month of testing for coronavirus disease 2019 (COVID-19) by using a nucleic acid amplification test (NAAT) on nasopharyngeal swabs at the Johns Hopkins Hospital, Baltimore, Maryland (11 066 persons).
Participants: Of the 11 066 tested persons, 115 (1%) were hospitalized adults investigated for COVID-19. Clinical record review was performed to classify them into a COVID-19 case group (n = 60) or a non-COVID-19 control group (n = 55). The laboratory control groups comprised 513 persons not tested by NAAT: 160 healthy laboratory employees, 101 persons positive for IgG antibodies against Epstein-Barr virus capsid antigen, 215 positive for thyroperoxidase antibody, and 37 positive for rheumatoid factor.
Measurements: Serum IgG and IgA antibodies against SARS-CoV-2 spike protein were detected by using enzyme-linked immunosorbent assay.
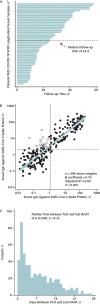
Results: Sensitivity and specificity of the SARS-CoV-2 IgG assay were 0.976 (95% CI, 0.928 to 0.995) and 0.988 (CI, 0.974 to 0.995), respectively, when performed 14 days or later after symptom onset, but sensitivity decreased at earlier time points. Immunoglobulin G developed rapidly and was sustained at high levels throughout follow-up (up to 58 days). Antibodies to SARS-CoV-2 predicted the odds of developing acute respiratory distress syndrome, which increased by 62% (CI, 48% to 81%; P < 0.001) for every 2-fold increase in IgG. Of 11 066 NAAT-tested patients, 457 were repeatedly NAAT-negative, and serum samples were obtained for 18 such patients (6 COVID-19 case patients and 12 non-COVID-19 control patients). Antibodies were present in 5 of 6 case patients and none of the 12 control patients (P = 0.001).
Limitations: The study was retrospective and performed at a single center; the sample was small; follow-up was limited; and selection bias may have occurred.
Conclusion: Antibodies to SARS-CoV-2 demonstrate infection when measured at least 14 days after symptom onset, are associated with clinical severity, and provide valuable diagnostic support in patients who test negative by NAAT but remain clinically suspicious for COVID-19.
Primary funding source: Clinical Immunology Laboratory, Department of Pathology, Johns Hopkins Hospital.
Conflict of interest statement
Figures


References
-
- National Center for Health Statistics, Centers for Disease Control and Prevention. COVID-19 pandemic planning scenarios. 20 May 2020. Accessed at www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html on 1 June 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
