Winter is coming: Pathogen emergence in seasonal environments
- PMID: 32628658
- PMCID: PMC7365480
- DOI: 10.1371/journal.pcbi.1007954
Winter is coming: Pathogen emergence in seasonal environments
Abstract
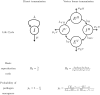
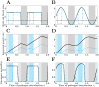
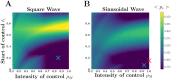
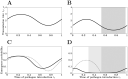
Many infectious diseases exhibit seasonal dynamics driven by periodic fluctuations of the environment. Predicting the risk of pathogen emergence at different points in time is key for the development of effective public health strategies. Here we study the impact of seasonality on the probability of emergence of directly transmitted pathogens under different epidemiological scenarios. We show that when the period of the fluctuation is large relative to the duration of the infection, the probability of emergence varies dramatically with the time at which the pathogen is introduced in the host population. In particular, we identify a new effect of seasonality (the winter is coming effect) where the probability of emergence is vanishingly small even though pathogen transmission is high. We use this theoretical framework to compare the impact of different preventive control strategies on the average probability of emergence. We show that, when pathogen eradication is not attainable, the optimal strategy is to act intensively in a narrow time interval. Interestingly, the optimal control strategy is not always the strategy minimizing R0, the basic reproduction ratio of the pathogen. This theoretical framework is extended to study the probability of emergence of vector borne diseases in seasonal environments and we show how it can be used to improve risk maps of Zika virus emergence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Woolhouse ME. Population biology of emerging and re-emerging pathogens. Trends in microbiology. 2002;10(10):s3–s7. - PubMed
-
- Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton University Press; 2011.
-
- Diekmann O, Heesterbeek H, Britton T. Mathematical tools for understanding infectious disease dynamics Princeton Series in Theoretical and Computational Biology. Princeton University Press, Princeton, NJ; 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

