Enhanced oxidative stress and damage in glycated erythrocytes
- PMID: 32628695
- PMCID: PMC7337333
- DOI: 10.1371/journal.pone.0235335
Enhanced oxidative stress and damage in glycated erythrocytes
Abstract
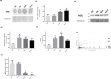
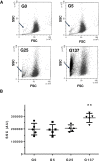
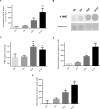
Diabetes is associated with a dramatic mortality rate due to its vascular complications. Chronic hyperglycemia in diabetes leads to enhanced glycation of erythrocytes and oxidative stress. Even though erythrocytes play a determining role in vascular complications, very little is known about how erythrocyte structure and functionality can be affected by glycation. Our objective was to decipher the impact of glycation on erythrocyte structure, oxidative stress parameters and capacity to interact with cultured human endothelial cells. In vitro glycated erythrocytes were prepared following incubation in the presence of different concentrations of glucose. To get insight into the in vivo relevance of our results, we compared these data to those obtained using red blood cells purified from diabetics or non-diabetics. We measured erythrocyte deformability, susceptibility to hemolysis, reactive oxygen species production and oxidative damage accumulation. Altered structures, redox status and oxidative modifications were increased in glycated erythrocytes. These modifications were associated with reduced antioxidant defence mediated by enzymatic activity. Enhanced erythrocyte phagocytosis by endothelial cells was observed when cultured with glycated erythrocytes, which was associated with increased levels of phosphatidylserine-likely as a result of an eryptosis phenomenon triggered by the hyperglycemic treatment. Most types of oxidative damage identified in in vitro glycated erythrocytes were also observed in red blood cells isolated from diabetics. These results bring new insights into the impact of glycation on erythrocyte structure, oxidative damage and their capacity to interact with endothelial cells, with a possible relevance to diabetes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

