In Vivo Single-Molecule Detection of Nanoparticles for Multiphoton Fluorescence Correlation Spectroscopy to Quantify Cerebral Blood Flow
- PMID: 32628854
- PMCID: PMC8405275
- DOI: 10.1021/acs.nanolett.0c02280
In Vivo Single-Molecule Detection of Nanoparticles for Multiphoton Fluorescence Correlation Spectroscopy to Quantify Cerebral Blood Flow
Abstract
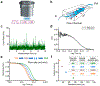
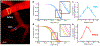
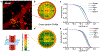
We present the application of multiphoton in vivo fluorescence correlation spectroscopy (FCS) of fluorescent nanoparticles for the measurement of cerebral blood flow with excellent spatial and temporal resolution. Through the detection of single nanoparticles within the complex vessel architecture of a live mouse, this new approach enables the quantification of nanoparticle dynamics occurring within the vasculature along with simultaneous measurements of blood flow properties in the brain. In addition to providing high resolution blood flow measurements, this approach enables real-time quantification of nanoparticle concentration, degradation, and transport. This method is capable of quantifying flow rates at each pixel with submicron resolution to enable monitoring of dynamic changes in flow rates in response to changes in the animal's physiological condition. Scanning the excitation beam using FCS provides pixel by pixel mapping of flow rates with subvessel resolution across capillaries 300 μm deep in the brains of mice.
Keywords: Multiphoton in vivo imaging; cerebral blood flow (CBF); fluorescence correlation spectroscopy (FCS); nanoparticles.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

