Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits
- PMID: 32629187
- PMCID: PMC7309780
- DOI: 10.1016/j.jcv.2020.104519
Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits
Abstract
Background: The severe shortage of nucleic acid extraction kits during the current COVID-19 pandemic represents a key limiting factor in testing capacity.
Objectives: This study compared the results of SARS-CoV-2 RT-PCR using different simple nucleic acid extraction methods on nasopharyngeal and saliva specimens.
Study design: Fifty nasopharyngeal swab and saliva specimens previously tested positive for SARS-CoV-2 were retrieved. Three different methods of nucleic acid extraction were compared. The first method involves incubating the specimen with proteinase K, and then heat treatment at 98 °C for 5 min (PKH); the second method involves heat treatment at 98 °C for 5 min without proteinase K pre-incubation (heat only); the third method involves no pre-processing steps (direct). The products from all 3 methods were tested by SARS-CoV-2 RT-PCR.
Results: PKH had significantly higher positive rate in SARS-CoV-2 RT-PCR (80 %) than those of heat only (58 %; P = 0.001) or direct (56 %; P = 0.002). The median Ct value was significantly earlier for PKH (median Ct: 37.0, IQR 31.7-40) than that of heat only (median Ct: 40, IQR 36.2-41; P < 0.0001) and direct (median Ct, 37.5; IQR 33.9-41.0; P = 0.0049). Subgroup analysis showed that PKH had higher detection rate, lower limit of detection and earlier Ct values than the other two groups for both NPS and saliva specimens.
Conclusions: PKH pre-processing resulted in the highest detection rate of SARS-CoV-2 by RT-PCR, and represents an alternative method for nucleic acid extraction when commercial extraction kits are not available.
Keywords: COVID-19; Detection; Nucleic acid extraction; Proteinase K; RT-PCR; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
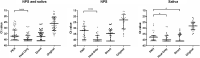
References
-
- Peto J. Covid-19 mass testing facilities could end the epidemic rapidly. BMJ. 2020;368:m1163. - PubMed
-
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31042-31044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

