Hematopoietic stem-cell senescence and myocardial repair - Coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT Phase 3
- PMID: 32629392
- PMCID: PMC7339012
- DOI: 10.1016/j.ebiom.2020.102862
Hematopoietic stem-cell senescence and myocardial repair - Coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT Phase 3
Abstract
Background: Bone marrow stem cell clonal dysfunction by somatic mutation is suspected to affect post-infarction myocardial regeneration after coronary bypass surgery (CABG).
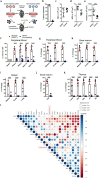
Methods: Transcriptome and variant expression analysis was studied in the phase 3 PERFECT trial post myocardial infarction CABG and CD133+ bone marrow derived hematopoetic stem cells showing difference in left ventricular ejection fraction (∆LVEF) myocardial regeneration Responders (n=14; ∆LVEF +16% day 180/0) and Non-responders (n=9; ∆LVEF -1.1% day 180/0). Subsequently, the findings have been validated in an independent patient cohort (n=14) as well as in two preclinical mouse models investigating SH2B3/LNK antisense or knockout deficient conditions.
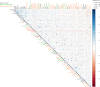
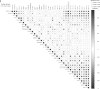
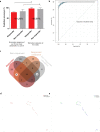
Findings: 1. Clinical: R differed from NR in a total of 161 genes in differential expression (n=23, q<0•05) and 872 genes in coexpression analysis (n=23, q<0•05). Machine Learning clustering analysis revealed distinct RvsNR preoperative gene-expression signatures in peripheral blood acorrelated to SH2B3 (p<0.05). Mutation analysis revealed increased specific variants in RvsNR. (R: 48 genes; NR: 224 genes). 2. Preclinical:SH2B3/LNK-silenced hematopoietic stem cell (HSC) clones displayed significant overgrowth of myeloid and immune cells in bone marrow, peripheral blood, and tissue at day 160 after competitive bone-marrow transplantation into mice. SH2B3/LNK-/- mice demonstrated enhanced cardiac repair through augmenting the kinetics of bone marrow-derived endothelial progenitor cells, increased capillary density in ischemic myocardium, and reduced left ventricular fibrosis with preserved cardiac function. 3.
Validation: Evaluation analysis in 14 additional patients revealed 85% RvsNR (12/14 patients) prediction accuracy for the identified biomarker signature.
Interpretation: Myocardial repair is affected by HSC gene response and somatic mutation. Machine Learning can be utilized to identify and predict pathological HSC response.
Funding: German Ministry of Research and Education (BMBF): Reference and Translation Center for Cardiac Stem Cell Therapy - FKZ0312138A and FKZ031L0106C, German Ministry of Research and Education (BMBF): Collaborative research center - DFG:SFB738 and Center of Excellence - DFG:EC-REBIRTH), European Social Fonds: ESF/IV-WM-B34-0011/08, ESF/IV-WM-B34-0030/10, and Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany. Japanese Ministry of Health : Health and Labour Sciences Research Grant (H14-trans-001, H17-trans-002) TRIAL REGISTRATION: ClinicalTrials.gov NCT00950274.
Keywords: Angiogenesis induction; CABG; CHIP; Cardiac stem cell therapy; Clonal hematopoiesis of indeterminate pathology; Coronary bypass surgery; Machine learning; Myocardial regeneration; Post myocardial infarction heart failure; SH2B3.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures





Comment in
-
Blood speaks: Personalised medicine profiling for heart failure patients.EBioMedicine. 2020 Aug;58:102900. doi: 10.1016/j.ebiom.2020.102900. Epub 2020 Jul 22. EBioMedicine. 2020. PMID: 32711252 Free PMC article. No abstract available.
References
-
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
-
- Korbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570–582. - PubMed
-
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

