Silencing of long non-coding RNA Sox2ot inhibits oxidative stress and inflammation of vascular smooth muscle cells in abdominal aortic aneurysm via microRNA-145-mediated Egr1 inhibition
- PMID: 32629426
- PMCID: PMC7377859
- DOI: 10.18632/aging.103077
Silencing of long non-coding RNA Sox2ot inhibits oxidative stress and inflammation of vascular smooth muscle cells in abdominal aortic aneurysm via microRNA-145-mediated Egr1 inhibition
Abstract
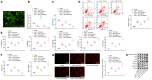
Long non-coding RNAs (lncRNAs) have been largely reported to contribute to the development and progression of abdominal aortic aneurysm (AAA), a common vascular degenerative disease. The present study was set out with the aim to investigate the possible role of lncRNA Sox2ot in the development of AAA. In this study, we found that lncRNA Sox2ot and early growth response factor-1 (Egr1) were highly expressed, while microRNA (miR)-145 was poorly expressed in Ang II-induced AAA mice and oxidative stress-provoked vascular smooth muscle cell (VSMC) model. Egr1 was a potential target gene of miR-145, and lncRNA Sox2ot could competitively bind to miR-145 to upregulate Egr1 expression. Overexpression of miR-145-5p was found to attenuate oxidative stress and inflammation by inhibiting Egr1 both in vivo and in vitro, which was counteracted by lncRNA Sox2ot. Taken together, the present study provides evidence that downregulation of lncRNA Sox2ot suppressed the expression of Egr1 through regulating miR-145, thus inhibiting the development of AAA, highlighting a theoretical basis for AAA treatment.
Keywords: Egr1; MicroRNA-145; abdominal aortic aneurysm; long non-coding RNA Sox2ot; oxidative stress.
Conflict of interest statement
Figures







References
-
- Liang ES, Bai WW, Wang H, Zhang JN, Zhang F, Ma Y, Jiang F, Yin M, Zhang MX, Chen XM, Qin WD. PARP-1 (Poly[ADP-ribose] polymerase 1) inhibition protects from ang II (Angiotensin II)-induced abdominal aortic aneurysm in mice. Hypertension. 2018; 72:1189–99. 10.1161/HYPERTENSIONAHA.118.11184 - DOI - PubMed
-
- Das S, Zhang E, Senapati P, Amaram V, Reddy MA, Stapleton K, Leung A, Lanting L, Wang M, Chen Z, Kato M, Oh HJ, Guo Q, et al.. A Novel Angiotensin II-Induced Long Noncoding RNA Giver Regulates Oxidative Stress, Inflammation, and Proliferation in Vascular Smooth Muscle Cells. Circ Res. 2018; 123:1298–312. 10.1161/CIRCRESAHA.118.313207 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

